The Sandmeyer reaction is a form of radical-nucleophilic aromatic substitution process. It’s a valuable tool for replacing an amino group on an aromatic ring with alternative substituents.
This reaction converts an aromatic amino group (NH2) into a very effective leaving group (N2), which can then be replaced by a variety of nucleophiles. However, because N2 is such a good leaving group, the process works best for aromatic amines; alkyl (“aliphatic”) amines tend to lose N2 too quickly, making the procedure ineffective.
Traugott Sandmeyer, a Swiss scientist, invented the reaction in 1884 while experimenting to synthesize phenylacetylene (C8H6) from benzene diazonium chloride (C6H5ClN2) and cuprous acetylide (C2CU2). However, as the major product, he obtained phenyl chloride.
The Sandmeyer reaction is an organic substitution reaction that is used to create an aryl halide from an aryl diazonium salt. A copper(I) halide catalyst, such as chloride, bromide, or iodide ions, is utilized in the Sandmeyer reaction.
What is Sandmeyer’s Reaction?
Benzene diazonium salt on heating with cuprous chloride or cuprous bromide in the presence of equivalent halogen acid from chlorobenzene and bromobenzene, resulting in the synthesis of aryl halide. This is known as the Sandmeyer reaction. This reaction can be used to substitute several substituents for an amino group on an aromatic ring.

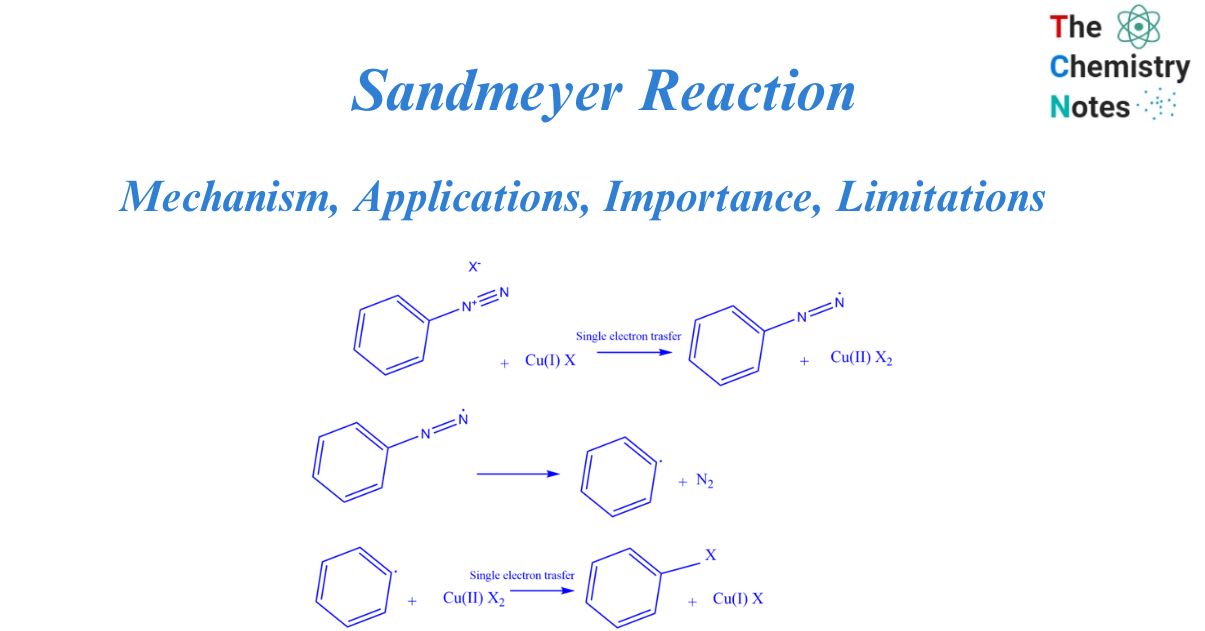
Mechanism of Sandmeyer reaction
A free radical mechanism governs the Sandmeyer reaction. The synthesis of aryl halides from primary aryl amines is a two-step process that involves the creation of diazonium salts and the transformation of diazo intermediates into aryl halides. The nucleophile can be a halide anion, cyanide, water, or other substance.
The Sandmeyer reaction starts with a single electron transfer from copper (catalyst) to diazonium. This produces a non-participating diazo radical as well as copper(II) halide. The diazo radical subsequently releases a molecule of nitrogen gas, producing an aryl radical, which then interacts with the copper(II) halide to restore the catalyst [copper(I) halide]. the aryl halide is obtained as the final product.
Mechanism of formation of Diazonium salt
When primary amine is treated with nitrous acid, the diazonium salt is formed, which is a compound of the type Ar/R-NN+ X–, where X- is an anion such as chloride, bromide, sulfate, and so on. However, because nitrous acid is unstable, it is produced in situ by treating sodium nitrite with a strong acid such as HCl or H2SO4. Diazotization is the process of converting primary amine to its diazonium salt.
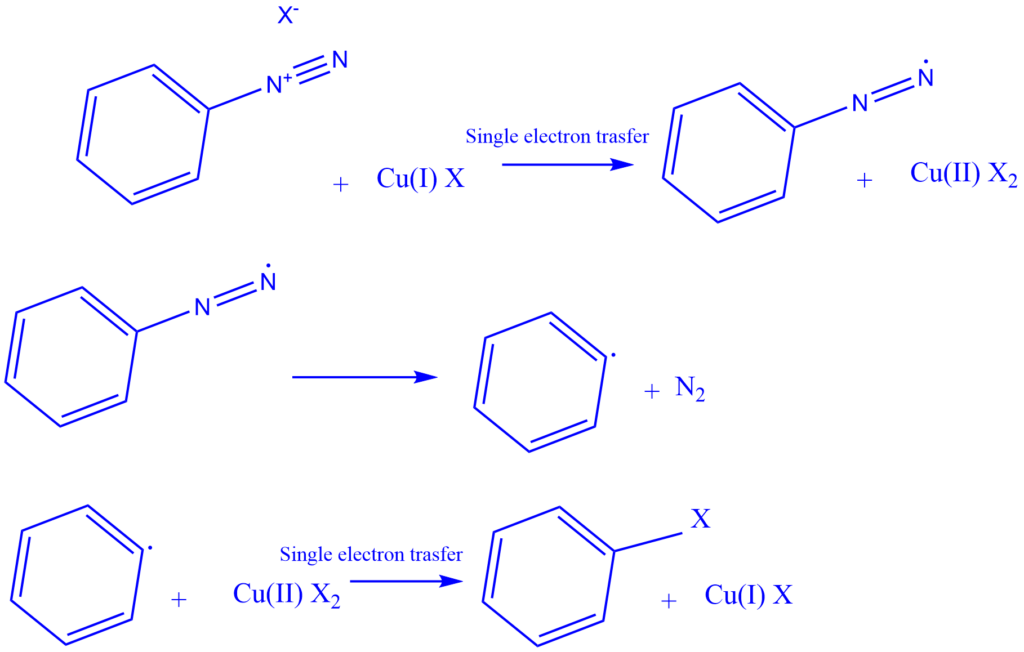
Generation of nitrosonium ion
The production method of diazonium salts involves the addition of NO, followed by a sequence of acid-base events that convert the oxygen into water and establish a triple bond between the two nitrogens. Afterward, the water is released as a leaving group.
The unstable nitrous acid must first be produced by treating sodium nitrite with aqueous HCl. Nitrous acid is formed by protonating a nitrite ion with HCl. The nitrous acid is then protonated to produce an oxonium ion with a suitable leaving group (water). Finally, the removal of the leaving group results in the formation of nitrosonium ion or nitrosyl cation.
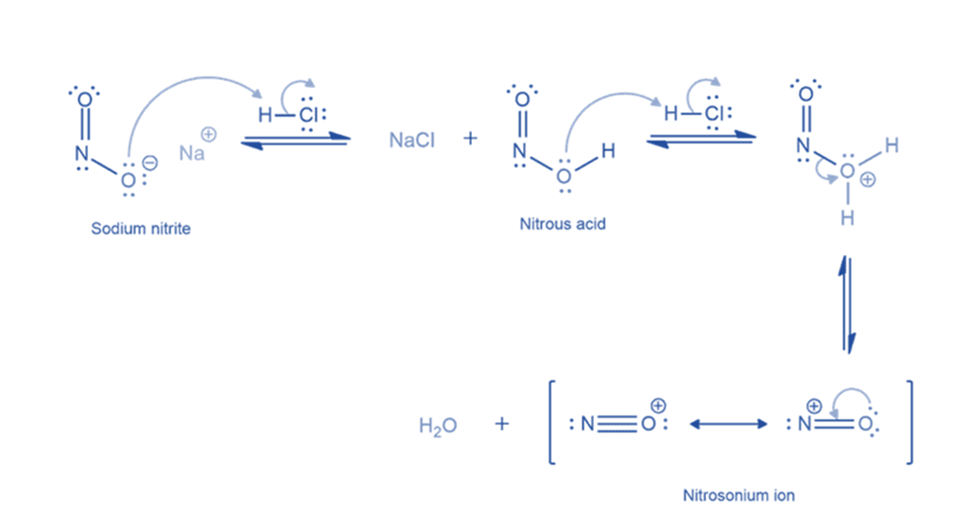
Formation of benzene diazonium ion
The nitrosonium ion is electrophilic, and amines can attack it to create an N-nitrosonium salt. When water deprotonates this nitrosammonium ion, a stable intermediate (N-nitrosamine) is generated. The oxygen atom of N-nitrosamine is then protonated, and water removes a proton from the nitrogen atom to form a double bond between the nitrogen atoms. The oxygen atom is protonated once more, resulting in a good leaving group (water). The departure of water results in the formation of a diazonium ion.
Applications of Sandmeyer reaction
- This reaction is used for the synthesis of aryl halides.
- This reaction is used for cyanation, which results in the creation of Benzonitriles, an important family of chemical compounds.
- Diazonium compounds have a wide range of applications in the dye and pigment industries. Initially, they were employed in the creation of water-fast colored fabrics.
- Diazonium salts have been discovered to be beneficial in the Fischer Indole Synthesis method because they may be converted to hydrazine derivatives using stannous chloride.
- They are used as standard reagents in the synthesis of organic molecules.
- It is utilized for the production of aryl compounds with trifluoromethyl groups, which are then used to make medicines.
- It is used to convert aryl amines into phenols.
Importance of Sandmeyer reaction
- The Sandmeyer reaction is critical when researching aromatic chemistry. The reaction can be used to generate substitution patterns that are not always available with direct substitution.
- In the procedure, a primary arylamine is diazotized to form an aryl diazonium salt, which is then reacted with a halide ion to yield the desired aryl halide product.
- This is based on an electron-transfer mechanism that incorporates free radicals.
- The Sandmeyer reaction has a wide range of synthetic applications and is beneficial in conjunction with electrophilic aromatic substitution.
Limitation of Sandmeyer reaction
- Because N2 is such a good leaving group, the process works best for aromatic amines; aliphatic amines tend to lose N2 too quickly, making the procedure ineffective.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
