An aromatic nucleophilic substitution reaction is the replacement of hydrogen or substituent on an aromatic ring by a nucleophile.
Aromatic rings do not undergo the aromatic nucleophilic substitution reaction under ordinary conditions. The reason for this low reactivity of the aromatic compounds having a nucleophile group as leaving group are as follows:
1. The lone pair of electrons or pi bonds on the atom of leaving group are involved in resonance with pi electrons of the aromatic ring and cannot be replaced easily.
2. Due to the presence of the electron cloud above and below the plane of the aromatic ring. This shields the ring carbon atom from the attack of the nucleophiles.
Interesting Science Videos
Types of nucleophilic substitution reaction mechanism
There are mainly four kinds of reactions that show the aromatic nucleophilic substitution reaction:
1. The reaction is activated by electron-withdrawing group ortho and para to leaving group.
2. Reaction that is catalyzed by a very strong base and proceeding through aryne intermediate.
3. The reaction initiated by electron donors.
4. Reaction that involves the replacement of nitrogen of a diazonium salt by a nucleophile.
There are four different types of the nucleophilic substitution reaction mechanism, which are similar to those of aliphatic nucleophilic substitution reactions
1. Substitution nucleophilic aromatic mechanism (SNAr)
2. SN1 mechanism
3. The benzyne mechanismm
4. SRN1 reaction
Substitution nucleophilic aromatic mechanism (SNAr)
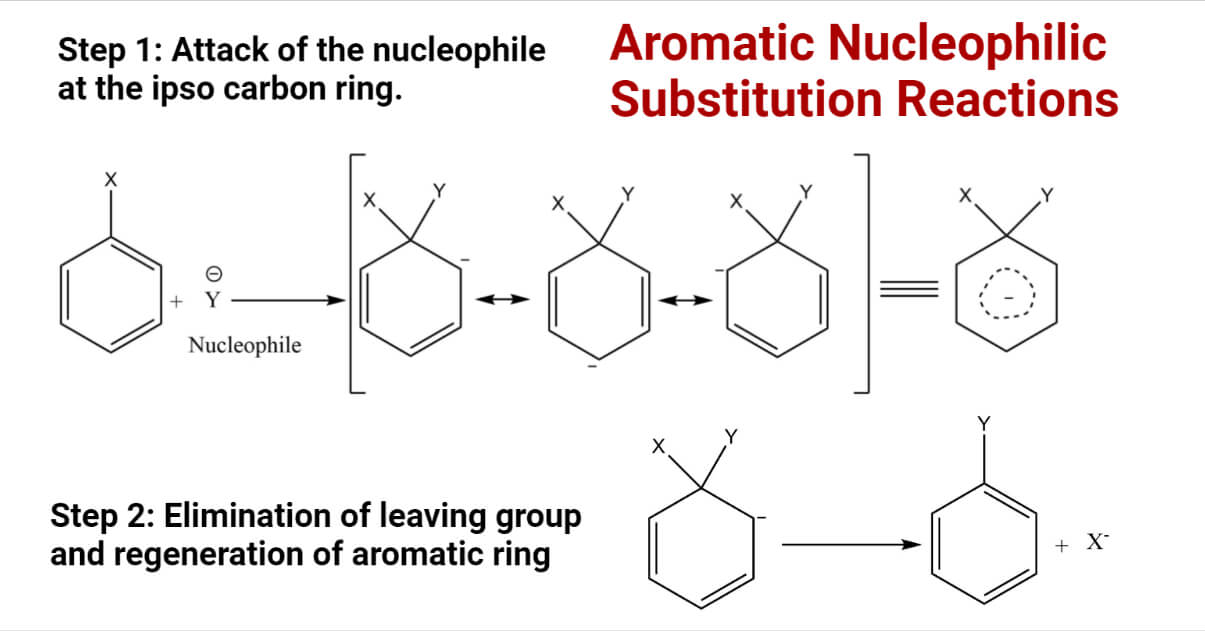
It is a reaction of addition and elimination. The SNAr reaction is very similar to the tetrahedral and arenium ion mechanisms of electrophilic aromatic substitution. In all three of these cases, the attacking species forms a bond with the substrate, producing an intermediate and then leaving the group departure. The most important mechanism of nucleophilic aromatic substitution consists of two-step:
Step 1: Attack of the nucleophile at the ipso carbon ring.

It is a rate-determining step in which the aromaticity of the benzene ring is lost.
Step 2: Elimination of leaving group and regeneration of aromatic ring

This step is fast, and the aromaticity of the benzene ring is restored in this step.
Evidence for Substitution nucleophilic aromatic (SNAr) mechanism
Meisenheimer salt has been isolated as an intermediate by the reaction between ethyl picrate and methoxide ions. NMR and X-ray crystallography have proved the structure of this intermediate.

SN1 mechanism
The SN1 reaction is rare and mainly involves the nucleophilic substitution reaction on aromatic diazonium salt.

Step 1: Formation of aryl cation

Step 2: Attack of a nucleophile

In this reaction, the aryl cation is highly unstable. Still, nitrogen is highly stable, so it is a very good leaving group, which makes the generation of the aryl cation extremely easy.
Evidence of SN1 mechanism
1. The rate of reaction is first order in the diazonium salt and independent of the nucleophile.
2. Addition of an excess amount of halide salt forms aryl halide is obtained, but the reaction is independent of the concentration of added salt.
3. The effect of ring substituents on the rate is consistent with the unimolecular rate-determining changes.
4. When Ar15N+ ≡N was used as the reaction species, the recovered starting material contained not only Ar15N+ ≡N but also ArN+ ≡N15. This could arise only if the nitrogen breaks away from the ring and then returns. This indicates that the 1st step is reversible cleavage.
The benzyne mechanism
The reactivity of the aryl halides, such as halobenzene re extremely low towards nucleophilic reagents under normal conditions. However, the substitution reaction occurs at high temperatures or in the presence of strong bases. So, the inactivated aryl halide having at least one hydrogen in the ortho position undergoes a substitution reaction in the presence of a strong base like KNH2, orNaNH2 in liquid ammonia. The reaction proceeds through the benzyne (aryne) intermediate, and the mechanism is called as benzyne mechanism.
For example, at 340°C, chlorobenzene reacts with sodium hydroxide to give phenol. It is an important commercial process for the production of phenol.

Furthermore, the conjugate bases of amines can convert aryl chlorides, bromides, and iodides to arene amines ArNH2. The incoming nucleophile does not always take the position vacated by the leaving group in this reaction. This is the interesting feature of this reaction.
Mechanism
This mechanism involves the elimination followed by addition so this reaction is also called the elimination addition mechanism of aromatic nucleophilic substitution reaction.
Step 1: Formation of benzyne intermediate

Step 2: Attack of a nucleophile

Evidence of benzyne mechanism
1. 1- 14C- chlorobenzene on treatment with potassium amide in liquid ammonia gives almost equal amounts of 1- 14C- aniline and 2- 14C- aniline. The formation of these two products can only be explained if the reaction is proceeding through a symmetrical intermediate which can attack by ammonia in either of two positions.

2. Aryl halide without ortho hydrogen does not react under the same condition.

3. Benzyne has been isolated in a matrix of argon at 8 K, where its IR spectrum can be observed. The order of halide reactivity is Br>I>Cl>F with NH2 in Liq NH3 shows that the SNAr mechanism is not operating here.
Prediction of major products in the reaction proceeding via benzyne intermediate
In the case of ortho and para-substituted halobenzene, two products are possible, whereas meta-substituted halobenzene gives three products. Two factors govern the position of the incoming group in a reaction involving benzyne intermediate. They are:
1. In the direction of benzyne intermediate
- In the case of ortho and para-substituted halobenzene, only one kind of benzyne intermediate is formed.

- Meta substituted halobenzene can form two different types of benzyne intermediate. In such cases, the more acidic hydrogen is removed i.e., if the substituent in halobenzene is a -I group then it will favor the removal of hydrogen ortho to it, whereas if the substituent in halobenzene is a +I group then it will favor removal of hydrogen para to it.

2. The nucleophile can attack the benzyne intermediate at two positions. The nucleophile mostly attacks the position, which gives stable carbanion. if the substituent in halobenzene is a -I group the most stable carbanion is that in which the negative charge is closer to the substituent.
The SRN1 mechanism
A unimolecular nucleophilic substitution reaction involving radical anions is called the SRN1 (substitution radical nucleophilic unimolecular) reaction. The single electron transfer (SET) from a chemical catalyst or an external electron source to the substrate initiates the SRN1 chain mechanism, resulting in the formation of a radical anion. The radical anion thus formed leads to the removal of leaving group and leaves behind a radical available for attack by the incoming nucleophile.
As radical anions are involved, SRN1 reactions can occur via a chain or a non-chain mechanism.
Reaction with an aryl halide

In this reaction, first, the aryl halide substrate accepts an electron from a donor. It becomes an anion radical (II), which losses the halide anion giving rise to an aryl radical (III). The aryl radical is easily attacked by the nucleophile to form a new radical anion (IV), which subsequently leads to product formation (V). The phenyl radical (III) can also abstract a proton from an alternate RH species resulting in chain termination.
5-iodo-1,2,4-trimethylbenzene on treatment with a strong base such as KNH2 in liquid NH3, two products were formed with a ratio of 0.63:1. There was the presence of a relatively less reactive substrate and strong base, so the reaction was expected to go by benzyne mechanism. However, the benzyne mechanism would have tended to result in an equal ratio of product formation, which was not observed here. As a result, a radical mechanism was first proposed for this reaction, which was confirmed by the addition of a radical scavenger, which results in different product ratios.

Evidence in support of the SRN1 mechanism
a. Addition of a good producer of solvated electrons increases the reaction.
b. Addition of radical scavengers suppresses the free radical mechanism.
c. Some amount of 1,2,4-trimthylbenzene was also observed along with the product which could be easily formed by the abstraction of H by Ar from solvent liquid NH3.
Factors affecting the reactivity in aromatic nucleophilic substitution
a. Effect of substrate
1. The presence of the electron-withdrawing group on the ortho and para position of leaving group increases the SNAr mechanism.
2. Heteroatoms, like nitrogen, of the ring increase the reaction rate.
3. The decreasing order of activating the power of some groups in SNAr reaction is
NH3+> NO2 > CF3 > CN > SO3H > CHO > CO > COOH > COOR > CONH2 > F > Cl > Br > I
b. Effect of leaving the group
In aliphatic nucleophilic substitution, the most common leaving groups are halide, sulfate, sulfonate, and NR3+. Some group like NO2, OR, OAr, SO2R, and SR also acts as leaving groups when attached to the aromatic rings. Depending on the intermediates formed in the rate-determining step, fluoro and nitro groups are good leaving groups in the SNAr reaction but poor leaving groups in the benzyne mechanism.
The decreasing order of leaving the group power is as follows:
F > NO2 > OTS > SOPh > Cl > Br > I > N3 > NR3+ > OAr, OR, SR, NH2
c. Effect of attacking nucleophile
As in the case of the aliphatic nucleophilic substitutions, nucleophilicity is generally dependent on basicity, and nucleophilicity increases as the attacking atom move down a column of the periodic table. The following is an approximate decreasing order of nucleophilicity.
NH2+ > Ph3C > PhNH+ > ArS– > RO– > R2NH > ArO– > OH– > ArNH2 > NH3 > I– > Br– > Cl– > H2O > ROH
References
- https://pubs.acs.org/doi/pdf/10.1021/cr60153a002
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/16%3A_Chemistry_of_Benzene__Electrophilic_Aromatic_Substitution/16.07%3A_Nucleophilic_Aromatic_Substitution
- https://byjus.com/chemistry/nucleophilic-aromatic-substitution/
- http://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/S000005CH/P000660/M004795/ET/s000005ch-p000660-m004795-et-v1.pdf
- https://www.masterorganicchemistry.com/2018/08/20/nucleophilic-aromatic-substitution-nas/
- https://www.sciencedirect.com/science/article/abs/pii/S0069804008701353
- https://www.mmcmodinagar.ac.in/econtent/chemistry/Reaction-mechanism.pdf
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)/18%3A_Reactions_of_Aromatic_Compounds/18.11%3A__NAS_Reactions_-the_Elimination-Addition(Benzyne)_Mechanism
