Nitro compounds are a type of organic compound distinguished by the presence of one or more nitro functional groups (NO2) directly attached to the carbon of the hydrocarbon chain or aromatic ring. Nitro compounds are typically created via a variety of synthetic ways, but a few of them occur naturally. Nitro compounds are represented by the general formula RNO2.
A nitro compound is a member of a chemical compound in which the nitro group (ON=O) is incorporated into the molecular structure. The most common examples are organic compounds with a covalent bond connecting a carbon atom to the nitrogen atom of the nitro group. Nitro compounds are polar, and those that do not contain another chemically reactive group are colorless or pale yellow liquids that are just slightly soluble in water.
Nitro compounds are highly explosive in nature, especially when more than one nitro group is present. Because of this, the nitro group is frequently classed as an explosophore group (a group that produces explosives). The explosive behavior of this group is related to the fact that when thermally decomposed, they yield molecular nitrogen as well as a significant quantity of energy. Trinitrophenol (picric acid), trinitrotoluene (TNT), and trinitroresorcinol (styphnic acid) are three of the most frequent explosive chemicals.
Interesting Science Videos
Classification of Nitro Compounds
Nitro compounds are hydrocarbon derivatives in which the nitro group replaces one or more hydrogen atoms. Depending on whether the nitro group is attached to an alkyl or an aryl group, they are further classed as aliphatic or aromatic nitro compounds.
Aliphatic Nitro Compounds
Aliphatic nitro compounds are formed when a nitro functional group is linked to an aliphatic carbon chain. Because of their azinitro form, they are slightly acidic, soluble in alkalies, and can undergo condensation reactions with aldehydes. For example:
CH3NO2 Nitromethane
Depending on whether the nitro group is linked to a primary, secondary, or tertiary carbon atom, they are further classed as primary, secondary, or tertiary.
Primary nitro alkanes: If the nitro group is attached to the primary carbon, then they are called primary nitroalkane.
Example: RCH2NO2
Secondary nitro alkanes: If the nitro group is attached to the secondary carbon, then they are called secondary nitroalkane.
Example: R2CHNO2
Tertiary nitro alkanes: If the nitro group is attached to the tertiary carbon, then they are called tertiary nitroalkane.
Example: R3CNO2
Nitro alkanes are alkane derivatives in which a nitro group replaces one or more hydrogen atoms. Nitroalkanes are employed as solvents in the production of oils, fats, shellac, and cellulose. Nitroalkanes are used in the manufacturing of oils, fats, shellac, and cellulose derivatives as solvents. They are utilized in the synthesis of primary amines, carboxylic acids, and their derivatives.
Aromatic Nitro Compounds
Aromatic nitro compounds are those that have a nitro functional group linked to a benzene ring. All aromatic nitro compounds are yellow liquids that oxidize to a dark brown color. For example:
C6H5NO2 Nitrobenzene
Structure of Nitro Group
The nitrogen group is thought to be a resonance hybrid (III) of two equivalent canonical structures I and II.

The nitro group’s sigma bond skeleton consists of three sp2 hybrid orbitals on nitrogen that create three sigma bonds–one with the carbon of the alkyl group and two with the oxygen atoms. However, nitrogen retains one unhybridized p orbital with an unshared electron pair, while each oxygen atom retains one half-filled p orbital. These three orbitals overlap sideways, resulting in a delocalized molecular orbital that includes all three atoms.
The nitrogen in the hybrid structure has a full positive charge and each oxygen has a half-negative charge. This corresponds to the high dipole moments of nitro compounds, which range between 3.5D and 4.0D depending on the type of R.
Because of the polar nature of the nitro group, nitro compounds have lower volatility than ketones of similar molecular weight; hence, the boiling point of nitromethane (MW 61) is 101o, whereas 2-propanone (MW 58) has a boiling point of 56o. Surprisingly, nitromethane has a low water solubility; a saturated solution in water contains less than 10% by weight, but 2-propanone is entirely miscible with water.
Nomenclature of Nitro Compounds
The name of any nitro compound is given by attaching the prefix nitro to the name of the corresponding alkane or the arene. Aliphatic nitro compounds are also known as nitroalkanes or nitroparaffins, while aromatic nitro compounds are known as nitroarenes.
Preparation of Nitro Compounds
From alkane
Direct nitration is a significant industrial process. This procedure primarily involves two techniques: liquid phase nitration and vapor phase nitration. The hydrocarbon is heated with strong nitric acid under pressure at 140 oC in liquid-phase nitration. Nitration is always slow under these conditions, and a substantial amount of poly-nitro compounds are formed. The hydrocarbon is heated with nitric acid or nitrogen oxides at 150-475 oC during vapor phase nitration. Vapor phase nitration is more effective than liquid phase nitration.

By the action of silver nitrite with alkyl halides
This approach is commonly used in the laboratory to prepare nitroalkanes. When alkyl halides are heated with silver nitrate, nucleophilic substitution occurs, resulting in nitroalkanes and minor amounts of alkyl nitrites. Nitro compounds are formed when iodo alkanes combine with silver nitrate. Nitroethane is produced when iodoethane is treated with silver nitrate.
CH3 – CH2– I + AgNO2 → CH3 – CH2– NO2 + AgI
Aromatic nitro compounds cannot be produced using this procedure due to aryl halide’s low reactivity to nucleophilic substitution. This approach is not suitable for large-scale production of nitro compounds.
From chloroacetate
Lower nitroalkanes, specifically nitromethane, are produced by reacting chloroacetic acid with sodium nitrite or potassium nitrite. Nitroacetic acid, which is generated as an intermediate product, decarboxylates to produce nitromethane.

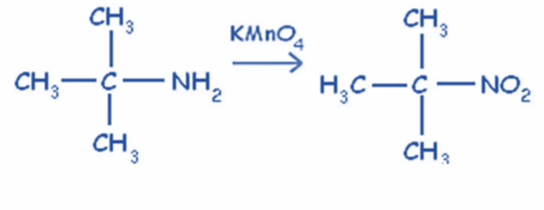
From tertiary amine
Tertiary amines in reaction with alkaline KMnO4 produce tertiary nitro compounds. This reaction only works with tertiary amines.
From oxidation of oximes
Trifluoroperacetic acid oxidizes aldoximes and ketoximes, forming 1o and 2o nitro compounds, respectively.

By direct nitration
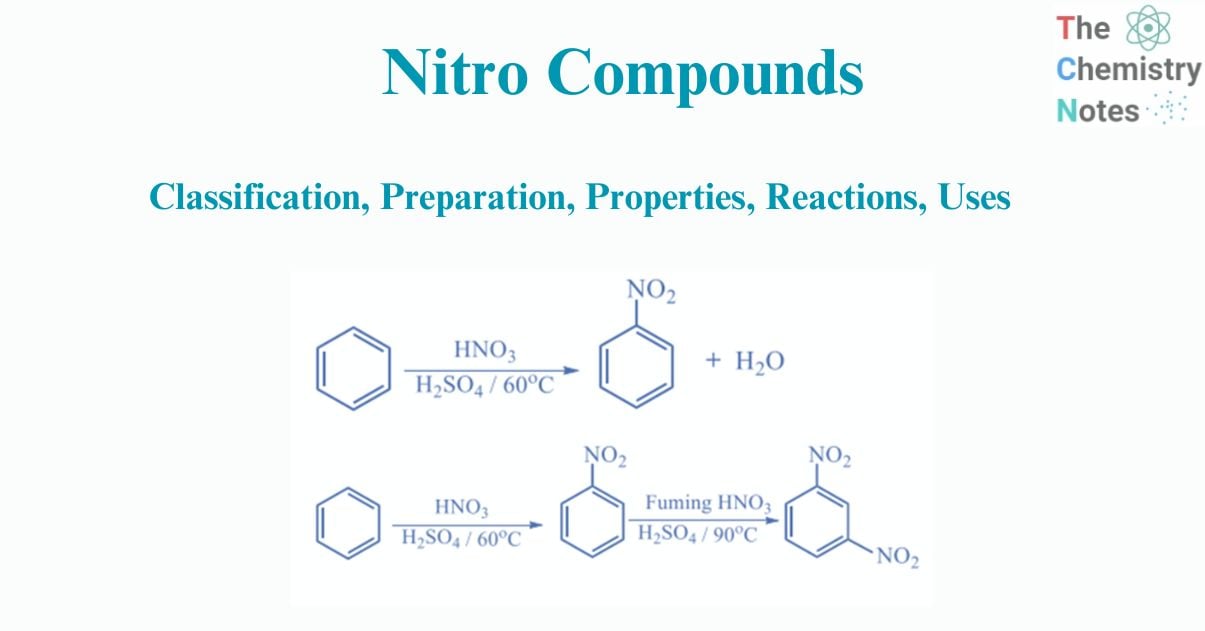
Aromatic hydrocarbons create nitro derivatives when they react directly with concentrated nitric acid or in the presence of a strong acid catalyst, such as sulfuric acid. This process is known as nitration, where the nitro group replaces hydrogen in the reaction.
Nitrobenzene is produced by nitration of benzene with “mixed acid,” a solution of strong sulfuric acid, water, and nitric acid.

From primary amines
Primary amines are oxidized with peracids in a sequence of steps to produce nitro compounds.

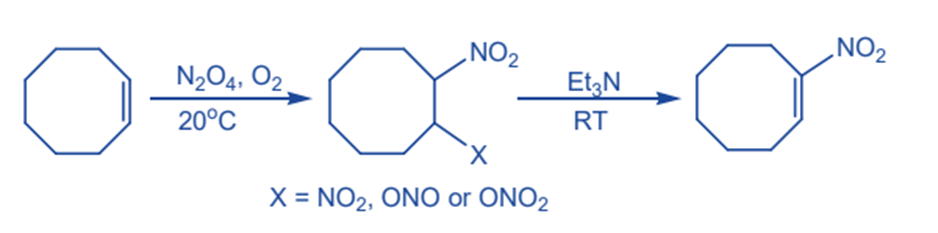
From unsaturated hydrocarbons
Alkenes can be successfully nitrated by dinitrogen tetraoxide in the presence of oxygen to produce a variety of vic dinitro compounds. Nitroalkene is produced as a result of a base-catalyzed elimination process.
From alkyl halides
Alkyl bromides or iodides on heating with lithium, potassium, or sodium nitrite in dimethylformamide or dimethylsulphoxide to produce primary and secondary nitroalkanes.

From arene diazonium compounds
When aromatic primary amines are diazotized with sodium nitrite and hydrogen borofluoride, arene diazonium salts are generated, which are then treated with copper powder and sodium nitrite to produce nitroarenes.

Properties of Nitro Compounds
- Nitroalkanes have a pleasant odor and are only slightly soluble in water.
- The vast majority of aliphatic nitro compounds are gaseous, while higher members are liquids.
- Aromatic nitro compounds are yellow liquids that darken to brown over time.
- Due to the presence of the nitro group, they are polar.
- Nitro compounds are more dense than water.
- Nitro compounds have a strong dipole-dipole interaction due to the presence of a highly polar nitro group. As a result, they have substantially higher boiling points than hydrocarbons of comparable molecular mass.
- Nitrobenzene is exceedingly poisonous in large quantities and is mostly manufactured as a precursor to aniline; however, it is occasionally utilized as a solvent in the laboratory, particularly for electrophilic reagents.
Isomerism in Nitro compounds
The different Isomerism exhibited by the nitro compounds is as follows:
- Chain isomerism
Chain isomers are compounds that have the same chemical formula but differ in the nature of their carbon chains. 1 nitro pentane and 2- methyl -1-nitro butane are chain isomers.
CH3 – CH2– CH2– CH2– CH2– NO2 1 nitro pentane
CH3 – CH2– CH(CH3)- CH2– NO2 2- methyl -1-nitro butane
- Positional isomerism
Position isomers are compounds that have the same chemical formula but differ in the position of attachment of the functional group. For example:
CH3 – CH2– CH2– CH2– CH2– NO2 1 nitro pentane
CH3 – CH2– CH2– CH (NO2) – CH3 2–nitro pentane
- Functional group isomerism
Nitro compounds are functional group isomers of nitrite compounds in which the oxygen is attached to the carbon chain. The representation below depicts the distinction between nitromethane and methyl nitrite.
CH3 – NO2 Nitro methane
CH3 – O-N=OMethyl nitrite
- Tautomerism
The nitro form is in equilibrium with the azinitro form in solution. Since the azinitro form is acidic in nature, nitromethane can be dissolved in alkalis.
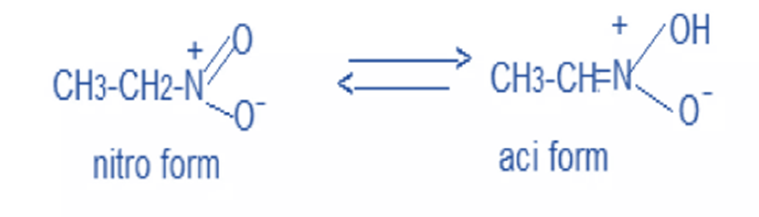
In solution, nitro compounds undergo tautomerism to generate azinitro, which is acidic in nature. As a result, all nitro compounds are weakly acidic in nature.
Reactions of Nitro compounds
Reduction
Many reagents reduce the nitrogen group extremely easily. Both aromatic and aliphatic nitro groups are easily reduced, and this can typically be done without damaging other functional groups. The behavior of the nitro group differs depending on whether it is attached to a carbon with no hydrogen or carbon with hydrogens. Tautomerism arises in the latter situation, which is decreased in various ways.
Reduction of aliphatic nitro compounds
When treated with Sn/HCl, aliphatic nitro compounds can be converted to amines. This is an efficient method for converting nitro compounds to amines.
CH3NO2 + Sn/HCl → CH3NH2
Lithium aluminum hydride and catalytic reduction using a transition metal catalyst are suitable ways for the complete reduction of the nitro group to yield primary amines.
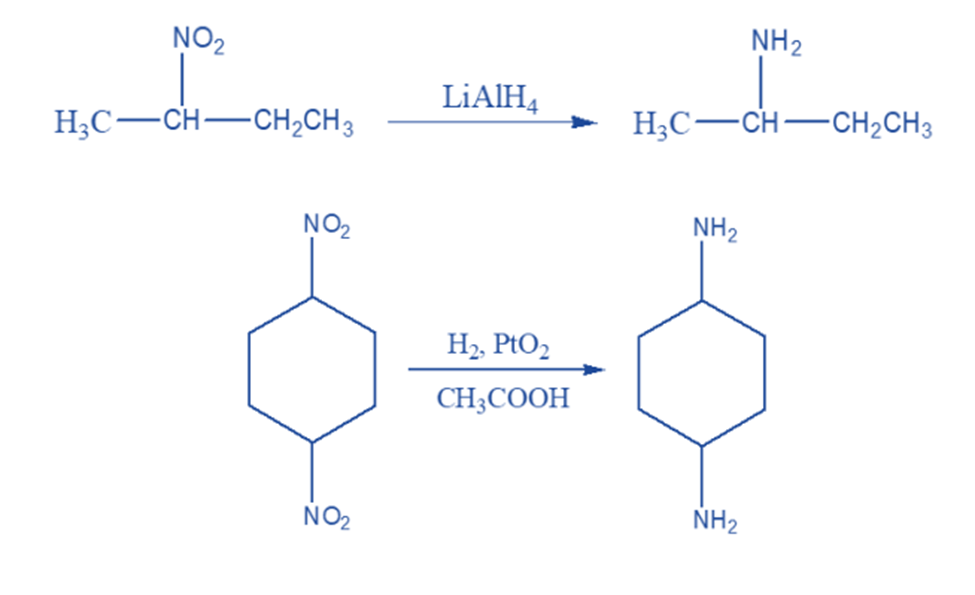
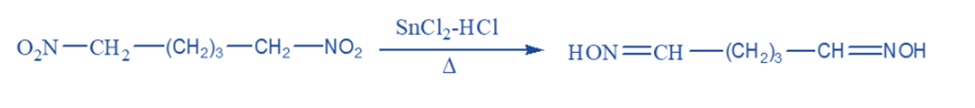
Metal salts such as stannous chloride and chromous chloride are commonly used to convert primary nitro compounds to aldoximes and secondary nitro compounds to ketoximes.
Catalytic reduction yields primary amine in an acid solution, with a Raney nickel catalyst producing 90-100%. Nitro compounds are transformed into hydroxylamine derivatives when reduced in a neutral solution, such as reduction with zinc dust and ammonium chloride in an aqueous or alcoholic solution.

Reduction of aromatic nitro compounds
Sn/HCI can convert nitro benzene to aniline.
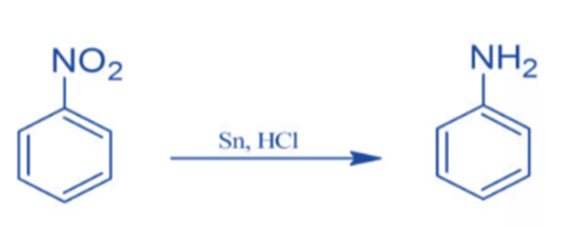
Nitrobenzene is converted to phenylhydroxylamine after being treated with Raney Nickel.

Catalytic hydrogenation with Pd on carbon and cyclohexene or triethylammonium formate enables partial reduction of dinitro compounds. Titanium trichloride, hydrogen sulfide in pyridine, and sodium or ammonium sulfide are other reagents for this.

Alkaline ferrous sulfate is a reliable reagent for selectively reducing nitro groups in the presence of carbon-carbon multiple bonds in the side chain.

Reductive removal of the nitro group
The nitro group can be removed from an aromatic ring by reducing it to amine, then deoxidizing it with HNO2and reductively removing the diazonium group with sodium borohydride or a hypo phosphorous acid /Cu+ mixture.

Hydrolysis
Boiling with hydrochloric acid or sulfuric acid hydrolyzes primary nitro compounds to carboxylic acid and hydroxylamine. This is the simplest and least expensive method of producing hydroxylamine.
R- CH2– NO2 + H2O + HCl → RCOOH + NH3OH
Secondary nitro compounds are hydrolyzed to ketones and nitrous oxide by boiling hydrochloric acid. Due to the lack of alpha hydrogen, tertiary nitro compounds are normally unaffected by hydrochloric acid.
2 R2CH-NO2 + H2O + HCl → 2 R2CO + N2O + H2O
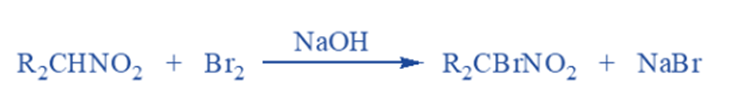
Halogenation reaction
In the presence of a base, primary and secondary nitroalkanes are easily halogenated with chlorine or bromine to generate chloro or bromo nitroalkanes. All alpha-hydrogens are replaced by halogens during the process. For example The bromination reaction,
Secondary nitro compounds can only create mono bromo derivatives, but primary nitro compounds can form both mono bromo and dibromo derivatives. Nitromethane is an uncommon compound capable of forming tribromo derivatives.
Reaction with aldehyde or ketone ( Henry reaction)
A reaction is analogous to the aldol reaction in which a carbon-carbon bond is generated by the reaction of a nitro molecule having alpha hydrogen (in aci form) with aldehyde or ketone to form -nitro alcohol. Nitro alcohol may also lose a water molecule, yielding alpha-nitroalkene.

Mannich reaction
Mannich reaction occurs in both primary and secondary nitro compounds. This is the reaction that occurs when formaldehyde, ammonia, or a 1o or 2o amine reacts with a substance that has at least one active hydrogen atom. An amino methyl group or a substituted amino methyl group replaces the active hydrogen atom.

Michael addition
α,β unsaturated esters and carbonyl compounds react with primary and secondary nitro alkanes (in their aci-nitro form) in an alcohol solvent in the presence of a basic catalyst such as sodium ethoxide or diethylamine to produce Michael addition product in high yield.

Nucleophilic substitution reaction
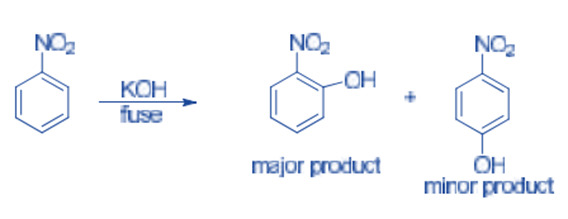
Nucleophiles readily attack the ortho and para positions of nitrobenzene. Nitrobenzene reacts with fused KOH to produce o-nitrophenol as the main product, with traces of p-nitrophenol produced.
Electrophilic aromatic substitution
Aromatic nitro compounds, like other aromatic compounds, are subject to electrophilic aromatic substitution processes such as nitration, sulphonation, and halogenation. However, the nitro group’s electron-withdrawing nature deactivates the ortho and para positions of the ring, making further electrophilic substitution reactions impossible. Because only one reactive site-meta is left after this selective deactivation, meta-substituted products are produced.

Alkylation
The anion formed as a result of nitroalkane deprotonation reacts with carbon electrophiles to produce a wide range of nitro-containing compounds. Deprotonation with butyllithium followed by a reaction with alkyl halide is a simple alkylation process.

Uses of Nitro Compounds
- They act as starting materials for organic synthesis reactions.
- They are useful intermediates in the synthesis of amino compounds and diazonium salts, which are essential ingredients in the synthesis of practically all organic molecules.
- In shoe and floor polishes, nitrobenzene is employed.
- It is also employed in paint and solvents, as well as for masking undesirable odors in chemicals.
- They are utilized in the preparation of azo dyes as well as soil sterility.
- It is used in the production of paracetamol (a common analgesic).
- Picric acid is an antiseptic as well as a dye for wool and silk.
- They are utilized in the production of explosives.
- They are used in laboratories to identify polynuclear hydrocarbons, amines, and alkaloids, among other compounds.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- http://cms.gcg11.ac.in/attachments/article/105/NITRO%20COMPOUNDS.pdf.
- https://www.britannica.com/science/nitro-compound.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Basic_Principles_of_Organic_Chemistry_(Roberts_and_Caserio)/24%3A_Organonitrogen_Compounds_II_-_Amides_Nitriles_and_Nitro_Compounds/24.06%3A_Nitro_Compounds
