Fischer Indole Synthesis: Mechanism, Features, Drawbacks
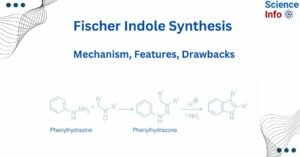
The Fischer indole synthesis is one of the oldest synthetic processes, and because of its versatility, it is commonly used to prepare substituted indoles. It was invented by Emil Fischer in 1883. … Read more