Aryl diazonium salt is the group of organic compounds characterized by the presence of function group -N≡N-directly bonded to the aryl group Ar. They have the general formula – ArN2+ X –, where X– may be an anion like Cl–, Br-, HSO4 –, BF4 – etc. Arenediazonium compounds are salts that are ionic in nature. This is reflected in their name, which is “Diazonium salts” (the word di refers to two, aza stands for nitrogen, and the final term onium reflects the compound’s ionic character). Thus Ionic compounds containing N≡N are referred to as diazonium salts.
Interesting Science Videos
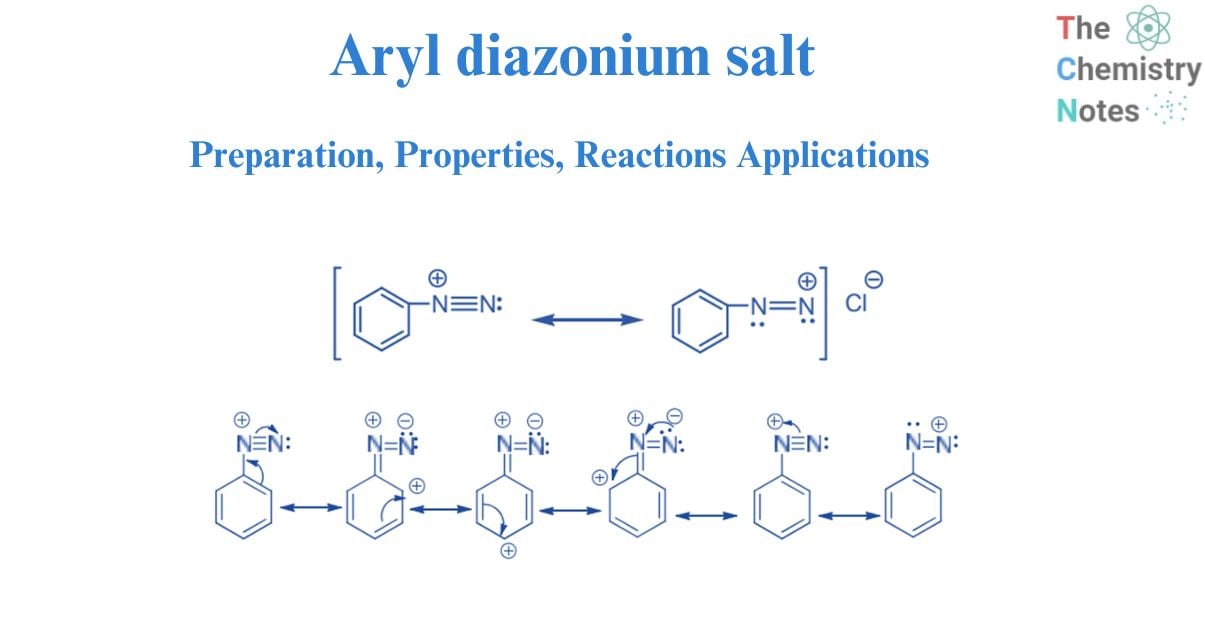
Nomenclature of diazonium salt
The diazonium salts are named by prefixing the word diazonium with the name of the parent aromatic chemical to which they are related, followed by the anion’s name. As an example,
Structure of diazonium salt
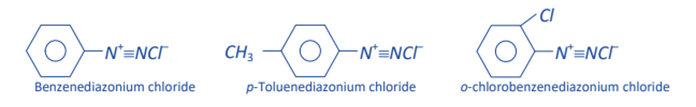
Benzene diazonium chloride can thus be represented by the following two electronic configurations, in which the positive charge is shared by both nitrogen atoms.
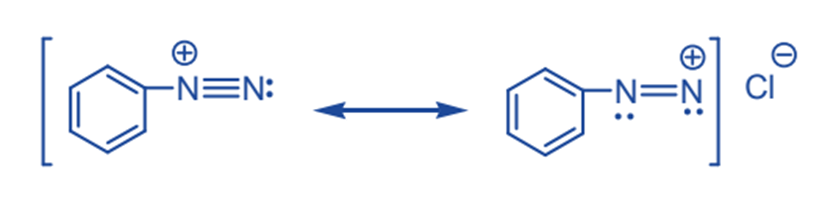
The relative stability of aromatic diazonium cation can be linked to the delocalization of the positive charge on nitrogen onto the ring’s pi system.
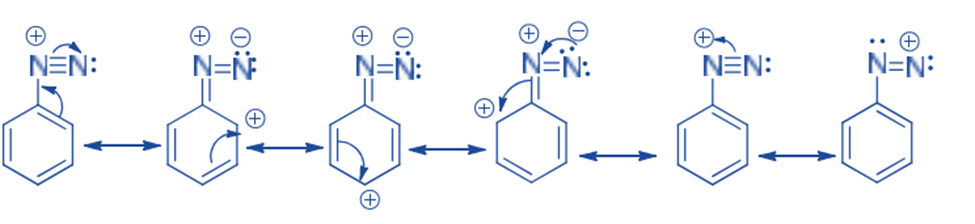
The positive charge of diazonium ions is delocalized over the two nitrogen atoms, according to a resonance structure. Nucleophiles cannot link to the inner nitrogen, while bonding (or coupling) nucleophiles to the terminal nitrogen produces neutral azo compounds. This coupling to the terminal nitrogen is a quick and reversible process.
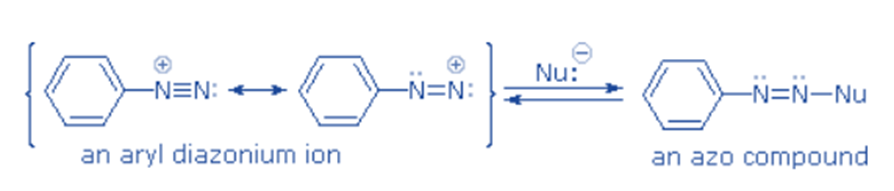
Unless these azo products are captured or stabilized in some way, the process will eventually reverse to the diazonium ion and undergo gradual nucleophilic substitution at carbon (with irreversible nitrogen loss). The azo products could have E / Z stereoisomers. In practice, the E-isomer is shown to predominate at equilibrium.
Preparation of diazonium salt
When the primary amine is treated with nitrous acid, diazonium salts are produced. As nitrous acid is highly unstable, it is generated in situ by adding sodium nitrite with a strong acid such as HCl or H2SO4. This process is known as diazotization.
NaNO2 + HCl→ HNO2 + NaCl
HNO2 + HNO2 → N+=O + H2O+ NO2–
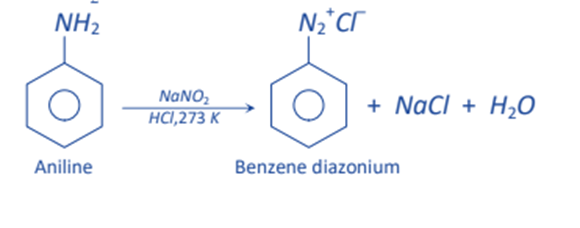
The majority of diazonium salts are stable below 5o C. As a result, it is essential to keep the reaction temperature below 5 oC, otherwise, the diazonium group will disintegrate to give N2 as soon as it forms.
Properties of diazonium salts
- Diazonium salts are colorless crystalline substances that darken when exposed to air.
- They are extremely soluble in water, but just slightly soluble in ethanol and insoluble in ether.
- Benzene diazonium chloride is water soluble but only reacts with it when heated.
- Benzene diazonium fluoroborate is insoluble in water. At room temperature, it is fairly stable.
- Many diazonium salts of nitrates and perchlorates explode when heated or struck when dry. As a result, these salts are not separated and are employed for additional synthetic preparations as soon as they are created in situ.
- Due to the presence of ions, their aqueous solutions conduct electricity and are neutral to litmus.
Reactions of diazonium salts
There are several synthetically beneficial reactions of Diazonium salts, however, there is a differentiation between those in which the nitrogen atoms of the diazonium group are lost and those in which they are maintained in the end product. Thus, the beneficial reactions of diazonium compounds can be divided into two categories.
(i) Reactions in which the diazo group is substituted by another functional group.
(ii) Reactions that retain the diazo group
Reactions in which the diazo group is substituted by another functional group.
Deamination reaction
Deamination is the replacement of a diazonium group by hydrogen, resulting in the overall elimination of an amino group. Certain mild reducing agents, such as hypophosphorous acid (phosphinic acid) or ethanol, convert diazonium ions to arenes, which are then oxidized to phosphorous acid and ethanal, respectively.
ArN2+X– + H3PO2 + H2O → ArH + N2 + H3PO3 + HX
This deamination of amines provides a valuable approach for the synthesis of trihalogen arenes that would otherwise be impossible to synthesize via direct halogenation of arenes. Direct halogenation of benzene produces a monohalogenated product; however, halogenation of aniline, which contains an activating amino group, guides halogenation to the ortho and para positions. The desired product is obtained by removing the amino group.
Replacement by a hydroxyl group
When aryl diazonium salt is heated with water or acid, it is hydrolyzed to form
The conditions needed to carry out the reaction are fairly mild, making this a more flexible technique for the synthesis of phenols. These phenols are crucial building blocks in medicines and medication development.
ArN2+X– + H2O → ArOH + N2 + H+
Replacement by NO2 group
Diazonium fluoroborate on heating with aqueous sodium nitrite solution in the presence of copper produces nitrobenzene. In this reaction, the diazonium group is replaced by the -NO2 group.
ArN2+Cl– + HBF4 → ArN2+ BF4– + NaNO2 → ArNO2 + N2 + NaBF4
Replacement by Halogen (Sandmeyer Reaction)
Freshly prepared diazonium salt solution on reaction with cuprous chloride or cuprous bromide produce haloarene. This reaction involves the replacement of the diazonium group by Cl or Br. Nitrogen is slowly created at room temperature or occasionally at higher temperatures, and the aryl chloride or aryl bromide can be separated from the reaction mixture after several hours. A cuprous halide-based approach for the formation of haloarene is known as the Sandmeyer reaction.
ArN2+X– + Cu2Cl2 /HCl → ArCl + N2
ArN2+X– + Cu2Br2 /HCl → ArBr + N2
ArN2+X– + CuCN / KCN → ArCl + N2
Replacement by fluorine- Balz-Schiemann reaction
The reaction of the diazonium salt with fluoroboric acid results in the formation of aryl diazonium fluoroborate salt. When heated, aryl diazonium fluoroborate decomposes to aryl cation intermediate, which promptly reacts with tetrafluoroborate to generate aryl fluoride and boron trifluoride.
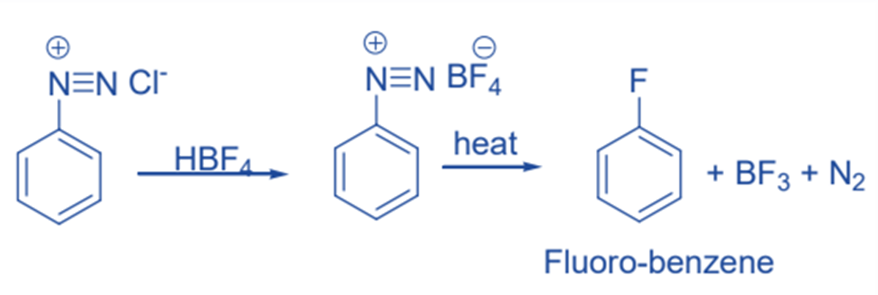
Replacement by -I group
Iodine is not easily inserted into the benzene ring directly, but iodobenzene is generated when the diazonium salt solution is treated with potassium iodide.
ArN2+Cl– + KI → ArI + N2 + KCl
Meerwein arylation
The reaction of aryldiazonium salt with alkene containing electron withdrawing groups such as COOH, COOR, NO2, etc. in the presence of copper (I) salt is known as Meerwein arylation.
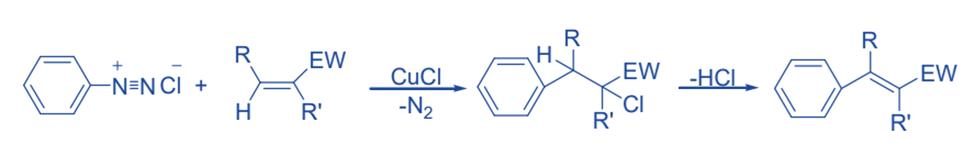
Gomberg-Bachmann reaction
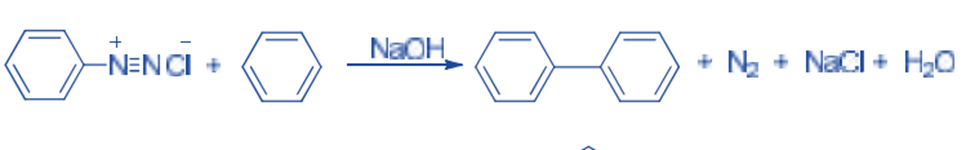
In the presence of dilute sodium hydroxide, diazonium salt reacts with an arene to give biaryl derivatives. This is known as the Gomberg-Bachmann reaction. For example, Benzene reacts with benzene diazonium chloride to give diphenyl.
Replacement by cyano group
The reaction which involves the replacement of the diazo group by the cyano group is a specific example of the Sandmeyers reaction, also known as the modified Sandmeyers reaction. In this reaction process, diazonium salt solution is treated with a solution of copper (I) cyanide in aqueous potassium cyanide to give a cyano derivative of benzene.
ArN2+X– → ArCN + N2
Hydrolysis of these nitriles produces carboxylic acid. As a result, producing nitrites from diazonium salts provides a quick way to convert nitro compounds to carboxylic acids.
Gattermann reaction
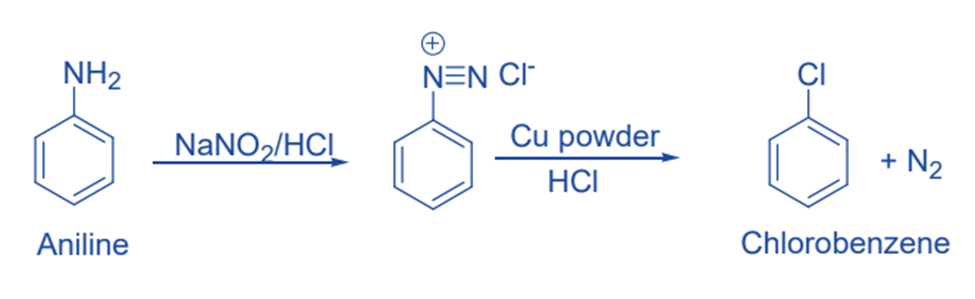
This reaction, named after German chemist Ludwig Gatterman, was originally used to synthesize aromatic aldehydes, but it is now being used to synthesize aryl halides from diazonium salt. The reaction is a variation of Sandmeyer’s reaction in which chlorine or bromine can also be introduced in the benzene ring by treating the diazonium salt solution with corresponding halogen acid in the presence of copper powder.
Bart reaction (replacement by arsenic group)
The reaction was named after H. Bart [German Patent-250264, 1910]. It involves the reaction of a diazonium compound with alkali arsenite, such as sodium arsenate, in the presence of cupric salt, powdered silver, or copper powder, with to form aryl arsonic acid.
C6H5N2+Cl– + Na3AsO3 → C6H5 AsO3 Na2 + NaCl + N2
C6H5 AsO3 Na2 + 2 HCl → C6H5 AsO3 H2 + 2 NaCl
Reactions that retain the diazo group
Reduction to arylhydrazines
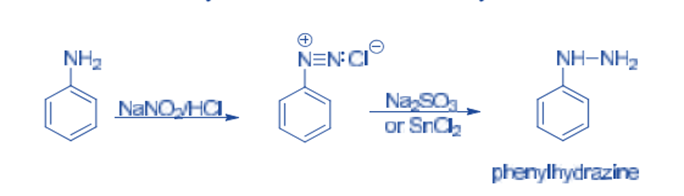
Arylhydrazines are made by reducing diazonium salts with sodium sulfite or stannous chloride, then acidifying them with hydrochloric acid. On cooling, the hydrochloride derivative crystallizes, which releases the free hydrazine derivative after treatment with sodium hydroxide.
Coupling reaction
Coupling reactions occur when diazonium salts join or are coupled with another molecule. The amine to be diazotized is known as the diazonium component, and the compound with which it is coupled is known as the coupling component. The entire process is known as azo coupling. Diazonium salts are weak electrophiles, and as a result, they react with aromatic systems activated by strong electron-donating groups. As a result, diazonium salts are typically coupled with phenols and aromatic amines in alkaline, neutral, or mild acidic media. Diazonium salts easily react with phenols, naphthols, and aromatic amines to form colored azo compounds. The -N=N- bond joins both aromatic rings in azo products, resulting in an extended conjugated system. These compounds are usually colored. This sort of connection is also known as C-coupling. For example:
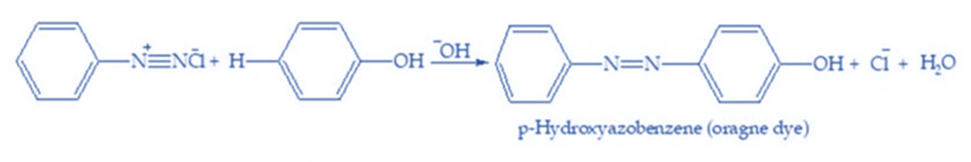
When benzene diazonium chloride interacts with phenol, the para position of the phenol molecule is connected to the diazonium salt, resulting in p-hydroxy azobenzene.
Applications of diazonium salts
- Diazonium salts are frequently utilized in the pigment and dye industries, particularly in the production of colored textiles.
- Diazonium salts are frequently used to make azo colors. As a result, they are critical in industrial and synthetic organic chemistry.
- Diazonium compounds, particularly aryl derivatives, are common reagents in organic molecule synthesis.
- They are employed as intermediates in the aromatic ring to introduce -F, -Br, -Cl, -I, -NO2, -OH, and -CN groups.
- They are employed in document reproduction due to their ability to degrade when exposed to UV light. In this method, paper or film is coated with diazonium salt. After contact exposure to light, the remaining diazo is converted to a stable azo dye using an aqueous solution of coupler.
- since the colors of the conjugate acid and conjugate base differ. It is possible to use azo compounds as indicators since they have both an acidic and a basic group.
- Methyl orange and methyl red, for example, are produced by combining dimethylaniline with diazotized sulfanilic acid and diazotized anthranilic acid, respectively.
- In a diazonium salt nanotechnology application, 4-chlorobenzene diazonium tetrafluoroborate is particularly effective at functionalizing single-wall nanotubes.
- Direct halogenation cannot produce aryl fluorides or iodides. The cyano group cannot be introduced into chlorobenzene via nucleophilic replacement of chlorine, while cyanobenzene can be easily produced from diazonium salt.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- https://www.masterorganicchemistry.com/2018/12/03/reactions-of-diazonium-salts-sandmeyer-and-related-reactions/.
- https://byjus.com/chemistry/diazonium-salts-application/.
- https://unacademy.com/content/jee/study-material/chemistry/diazonium-salts-importance-in-synthetic-organic-chemistry/.
- https://www.spiroacademy.com/pdf-notes/study-meterials/Chemical/nitro-compounds.pdf
