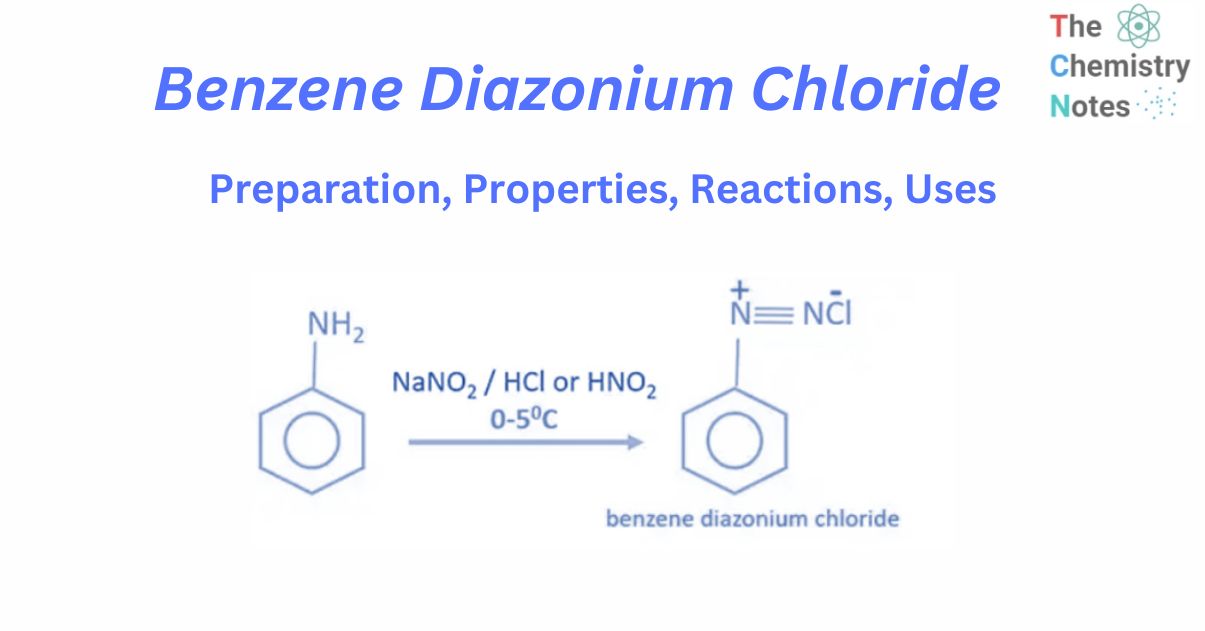
Benzene diazonium chloride is an organic compound with the chemical formula C6H5N2+Cl–. It is an important benzene-containing molecule because it can be used to create a wide range of other chemicals, including halobenzenes. Benzene diazonium chloride is a colorless solid with numerous applications in organic chemistry.
Preparation of Benzene Diazonium Chloride
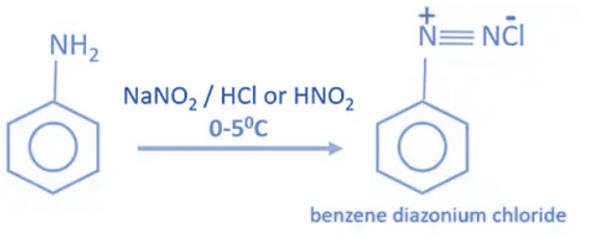
Aniline reacts with sodium nitrate or NaNO2 and hydrochloric acid or HCl at 0 – 5°C to form benzene diazonium chloride or Diazonium salt. When aniline interacts with nitrous acid at low temperatures (0-50 C), benzene diazonium chloride is obtained as the final product. When the temperature rises, benzene diazonium chloride decomposes into phenol. As a result, when making benzene diazonium chloride, temperature control is crucial.
Physical Properties of Benzene Diazonium Chloride
1. Diazonium salts are crystalline, colorless solids.
2. These are easily dissolved in water but less so in alcohol.
3. They are fragile and can explode when dried. As a result, they are typically employed in the solution state.
4. Because of the presence of ions, their aqueous solutions are litmus neutral and conduct electricity.
Chemical properties of Benzene Diazonium Chloride
Replacement by -H
A variety of reducing agents, including hypophosphorous acid, can be used to replace the diazonium group with H. Allowing the diazonium chloride to react in the presence of hypophosphorous acid leads to nitrogen loss and hypophosphorous acid oxidation to phosphorous acid.
ArN2+Cl– + H3PO2 + H2O → ArH + N2 + H3PO3 + HCl
Replacement by -OH group
Benzene diazonium chloride reacts with water to form phenol. This reaction is slow in ice-cold diazonium salt solutions, which is why diazonium salts are employed immediately after synthesis; at higher temperatures, it can be made the primary reaction of diazonium chloride.
ArN2+Cl– + H2O → ArOH + N2 + H+
Coupling Reactions
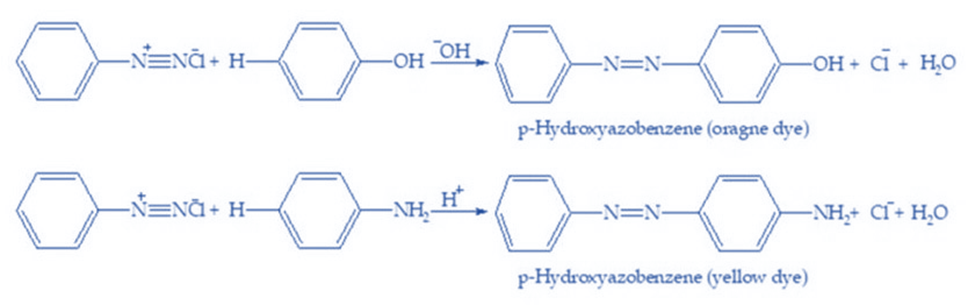
Diazonium chloride easily reacts with phenols, naphthols, and aromatic amines to form colored azo compounds. The -N=N- bond joins both aromatic rings in azo products, resulting in an extended conjugated system. These materials are frequently dyed and colored. When benzene diazonium chloride interacts with phenol, the para position of the phenol molecule is connected to the diazonium salt, yielding p-hydroxy azobenzene. This is referred to as a coupling reaction. Aniline also reacts with diazonium chloride to generate p-amino azobenzene. This is a promising electrophilic substitution technique.
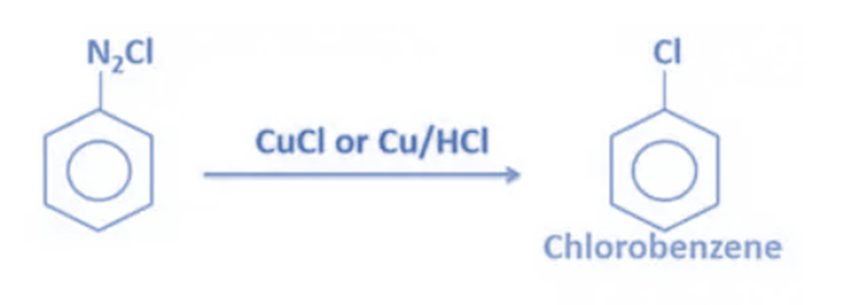
Sandmeyer reaction
- When benzene diazonium chloride is reacted with CuCl (Copper(I) chloride), chlorobenzene is produced. Instead of CuCl, copper powder with HCl can be used. Cu+ ion acts as a catalyst in this process.
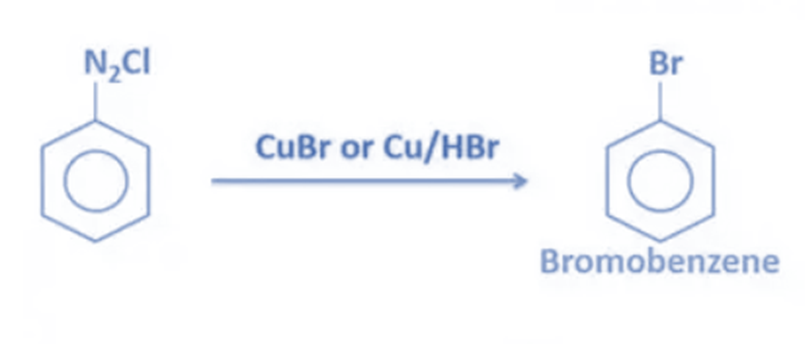
- When benzene diazonium chloride is reacted with CuBr (Copper(I) bromide), bromobenzene is produced. Instead of CuBr, copper powder with HBr might be used. Cu+ ion acts as a catalyst in this process.
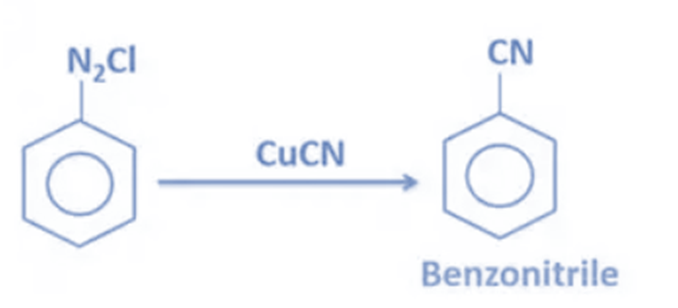
- Benzonitrile is formed when benzenediazonium chloride is reacted with CuCN (Copper(I) cyanide). Hydrolysis of these nitriles produces carboxylic acid. As a result, producing nitriles from diazonium salts provides a quick way to convert nitro compounds to carboxylic acids.
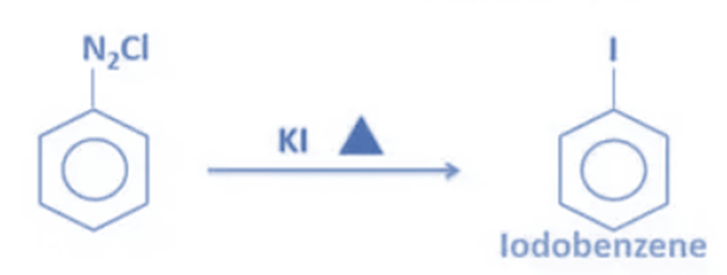
- The reaction between benzenediazonium chloride with KI (potassium iodide) can yield iodobenzene. However, the reaction medium must be heated. As with the preceding three reactions, a Cu+ catalyst is not required for this reaction.
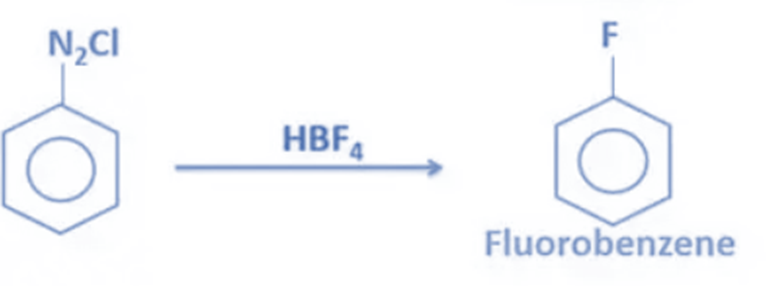
- Flurobenzene can be made by reacting benzenediazonium chloride with HBF4 (fluoroboric acid).
Gomberg-Bachmann reaction
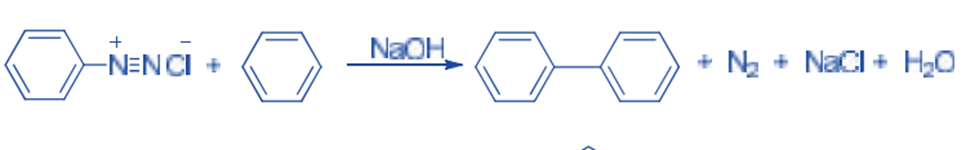
In the presence of dilute sodium hydroxide, diazonium salt reacts with an arene to give biaryl derivatives. This is known as the Gomberg-Bachmann reaction. For example, Benzene reacts with benzene diazonium chloride to give diphenyl.
Uses of Benzene Diazonium Chloride
- Many organic chemicals including benzene, nitrobenzene, and phenol are produced by using benzene diazonium chloride.
- They are used in the pigment and dye industries and the creation of colored fabrics.
- They are used in document reproduction because of their susceptibility to degrade when exposed to UV light.
- They are capable of producing a wide range of chemical compounds, particularly aryl derivatives. Straight halogenation is not even an option for preparing aryl iodides and fluorides. A cyano group cannot be substituted for the nucleophilic substitution of Cl in chlorobenzene.
Reference
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
