Sodium chloride is an essential mineral compound for human and animal health and has many industrial applications. Sodium chloride is another name for salt. It can be found in the seas and seawater. It’s also a component of rock salt.
Interesting Science Videos
What is Sodium Chloride?
Sodium chloride is an ionic compound in which the sodium and chloride ions are in equal amounts.
Sodium chloride (NaCl), or table salt, is a vital ingredient for many animals and plants as well as one of the most prevalent minerals on Earth. Seawater and igneous rock formations deep beneath both contain sodium chloride in their natural forms.
Both human and animal bodies need salt to function properly. The fine-grained and very pure salt used in cooking and seasoning is known as table salt. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium silicate are added to this hygroscopic (i.e. water-attracting) compound to keep it fluid when exposed to air. Iodized salt, or salt to which potassium iodide has been added in trace amounts, is routinely used in regions where iodine shortage leads to goitre (an enlargement of the thyroid gland). Salt is also needed by livestock, and it is often provided in the form of solid blocks.
Salt is used as a preservative or flavoring in the meat packing, sausage manufacturing, fish curing, and food processing industries. It’s used as a brine in fridges and for curing and preserving hides.
Occurrence of Sodium Chloride
Salts are the source of nearly all chemical compounds containing chlorine or sodium. It is widespread in the natural world. In seawater, salt is a significant component of the dissolved substances. Pure salt can be produced from the mineral halite. Mining the deposits results in the production of sodium chloride, while passing water through the deposits produces saline solution. In consequence, the salts dissolve, and the solution is poured out.
Seawater is the primary source of sodium chloride. It is one of the most commonly occurring minerals on Earth. Salt is also derived from salt deposits and salt brines. The United States, Great Britain, France, Germany, Russia, China, and India all contain significant deposits of this mineral. In tropical regions, it is produced by the natural evaporation of seawater. Approximately fifty million metric tons of salt are produced annually in India via sea salt harvesting.
Impurities in the form of calcium chloride, sodium sulfate, and magnesium chloride are present in the sodium obtained from the crystallization of brine. The coarse salt is dissolved in minute quantities of water in order to remove insoluble impurities. Then, the solution is saturated with hydrogen chloride gas.
Sodium Chloride can also be extracted from underground deposits using the room-and-pillar technique. By sinking passageways into the earth, miners use drilling and blasting to break up rock salt using the room and pillar method.
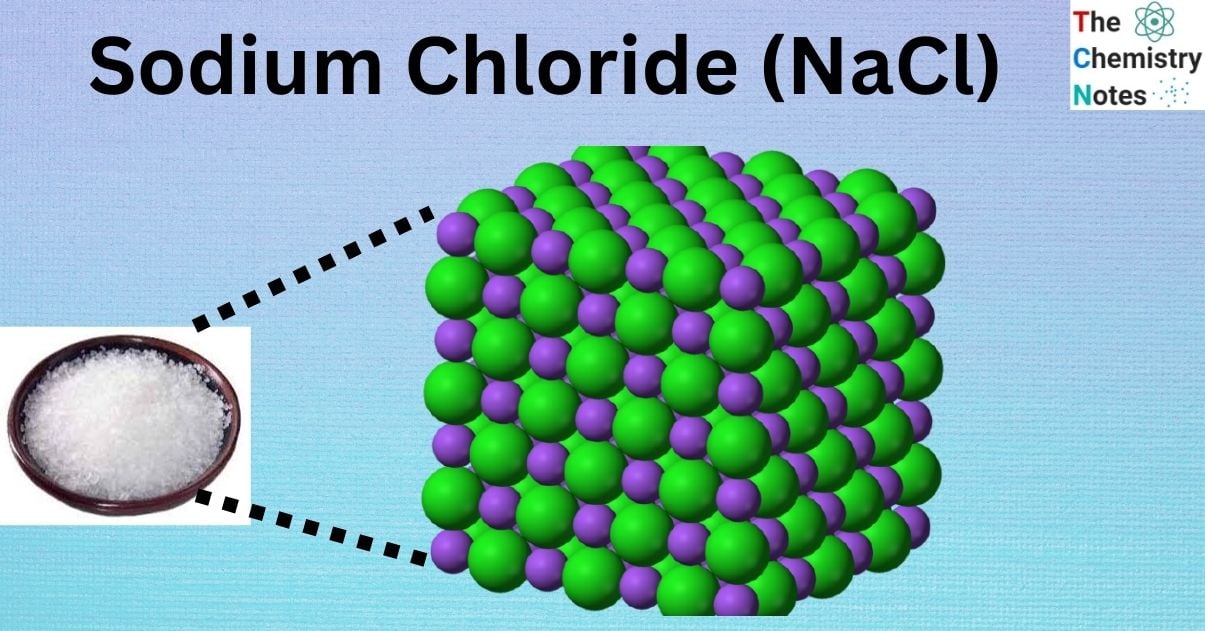
Structure of Sodium Chloride
Sodium chloride, or NaCl, is an ionic chemical with a huge, three-dimensional lattice structure that repeats indefinitely. Strong electrostatic forces of attraction keep the oppositely charged ions together in the enormous lattice. The anions are arranged in a face-centered cubic (fcc) array, and the cations are arranged in a face-centered cubic (fcc) lattice that is interpenetrating with the anions. Its geometry is localized as an octahedron.
![Structure of Sodium Chloride [Image Credit: Wikipedia]](https://scienceinfo.com/wp-content/uploads/2023/04/image-65.png)
The figure above illustrates the spatial relationships between Na+ and Cl– ions. Na+ ions are relatively tiny, having an atomic radius of 102 pm, while Cl– ions are rather massive, with an atomic radius of 181 pm. The coordination numbers of Na and Cl are identical because the ratio of Na to Cl in NaCl is exactly one-to-one. Cl– is depicted in green, whereas Na+ is shown in purple. Since the coordination numbers are similar, however, the structure of this molecule permits the locations to be exchanged.
Preparation of Sodium Chloride
At present, salt is produced in large quantities by evaporating seawater or brine from brine wells and salt lakes. The extraction of geological salt is another important source. China is the largest producer of salt in the globe. The mining of potassium also produces salt as a byproduct. Sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet.
2Na(s) + Cl2(g) → 2NaCl(s)
Physical Properties of Sodium Chloride
- It is readily soluble in water but either partially soluble or inert in other liquids.
- They are odorless but flavorful crystals that are white in color.
- Due to the unrestricted mobility of the ions, NaCl in its aqueous form is an excellent electrical conductor.
- Its melting point is 801°C, while its boiling point is 1,413°C.
- Sodium chloride has a +1 sodium oxidation state and a -1 chlorine oxidation state. The oxidation state of an atom is determined by the number of electrons it acquires or loses when it forms a compound. Sodium loses an electron, so its oxidation state is +1, while chlorine gains an electron, resulting in a -1 oxidation state.
- As its ions are equal in a solution, sodium chloride is neither an acid nor a base; it is a neutral compound.
- The crystal structure of sodium chloride is composed of a face-centered cubic lattice. The lattice constant of the material is 564.02 pm.
- Each sodium atom is surrounded by six chloride ions, and vice versa, due to the octahedral geometry of sodium chloride.
- The ionic nature of sodium chloride causes it to be a polar compound. Between Sodium and chloride, a dipole moment with a value of 8.5 D can be observed.
- Sodium chloride is a diamagnetic substance. Since all of the electrons in sodium and chlorine are paired. It cannot be paramagnetic because there are no unpaired electrons.
- There are no hydrates produced by sodium chloride. Since sodium chloride dissolves readily in water and does not form hydrates when re-crystallized from water, it is not possible to crystallize sodium chloride.
- The viscosity of sodium chloride can be determined because viscosity is an intrinsic property of fluids. By increasing the concentration of salt in water, the viscosity of the salt solution is observed to decrease.
Chemical Properties of Sodium Chloride
- Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. Sodium chloride also reacts with nitric acid to produce sodium nitrate and hydrogen chloride. When sodium chloride and hydrogen chloride are combined, no reaction is observed.
2NaCl + H2SO4 → Na2SO4 + 2HCl
NaCl + HNO3 → NaNO3 + HCl
NaCl + HCl → No noticeable reaction
- When sodium chloride reacts with a base such as potassium hydroxide, sodium hydroxide and potassium chloride are produced. However, there is no visible reaction when reacted with another base, sodium hydroxide.
NaCl + KOH → Na2O + MgCl2
- Sodium chloride reacts with an oxide of a metal, such as magnesium oxide, to create sodium oxide and magnesium chloride.
2NaCl + MgO → Na2O + MgCl2
- When sodium chloride reacts with metals, the most active metal will substitute for sodium to produce the corresponding metal chloride. The term for this is displacement reaction.
- In the industrial Solvay process, limestone (CaCO3) is utilized to generate carbon dioxide (CO2), which then reacts with ammonia (NH3) dissolved in concentrated sodium chloride (NaCl(aq)) to form sodium carbonates.
2NaCl + CaCO3 → Na2CO3 + CaCl2
Uses of Sodium Chloride
- It is utilized extensively in the culinary industry as a preservative and flavor enhancer.
- It is an essential basic material in the industrial production of a variety of compounds, including sodium carbonate, sodium hydrogen carbonate, etc.
- Numerous compounds, which devour the majority of the world’s production, are produced directly or indirectly with salt.
- Sodium chloride is used in the Solvay process to produce sodium carbonate and calcium chloride. Then, sodium carbonate is used to produce glass, sodium bicarbonate, dyes, and a number of other chemicals.
- Salt is an essential component of drilling fluids for effective oil and gas exploration drilling. It is used to flocculate and increase the density of the drilling fluid in order to counteract high gas pressures within the well. When a borehole encounters a salt formation, salt is added to the drilling fluid in order to permeate the solution and reduce dissolution within the salt layer.
- In textiles and dyeing, salt is utilized as a saline treatment to separate organic contaminants, promote salting out of dyestuff precipitates, and standardize concentrated dyes.
- Additionally, it is utilized in the production of aluminum, beryllium, copper, steel, and vanadium. Salt is utilized in the pulp and paper industry to neutralize wood fibers. It is also utilized in the production of sodium chlorate, which, when combined with sulfuric acid and water, yields chlorine dioxide, an excellent oxygen-based bleaching chemical.
- During tanning and leather treatment, salt is applied to animal hides to inhibit microbial activity on the underside of the hides and to draw moisture back into the hides.
- The production of buna, neoprene, and white rubber requires the use of salt. To coagulate a chlorinated butadiene latex emulsion, saline brine and sulfuric acid are utilized.
- Salt is also utilized to stabilize the soil and strengthen the foundations upon which highways are constructed.
- Due to its hygroscopic properties, sodium chloride is sometimes used as a low-cost and safe desiccant, making salting a viable method of food preservation.
- This salt is used in the production of glass.
- It is used in cold countries to prevent the accumulation of ice on roads, bridges, etc., which is essential for secure travel conditions.
Watch out the video about Sodium Chloride
References
- Westphal, Gisbert et al. (2002) “Sodium Chloride” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim doi:10.1002/14356007.a24_317.pub4
- Water-NaCl phase diagram. Lide, CRC Handbook of Chemistry and Physics, 86 ed (2005-2006), CRC pages 8-71, 8-116
- https://collegedunia.com/exams/sodium-chloride-definition-structure-properties-uses-chemistry-articleid-3495#Wh
- https://physicsopenlab.org/2018/01/22/sodium-chloride-nacl-crystal/
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_(Housecroft)/06%3A_Structures_and_Energetics_of_Metallic_and_Ionic_solids/6.11%3A_Ionic_Lattices/6.11A%3A_Structure_-Rock_Salt(NaCl)
- Chemistry/preparation-properties-and-uses-of-sodium-chloride/
- https://www.drugs.com/mtm/sodium-chloride-oral.html
- https://lambdageeks.com/sodium-chloride-properties/
- https://www.aakash.ac.in/important-concepts/chemistry/preparation-properties-and-uses-of-sodium-chloride

