Science Info is an educational niche website related to physics, chemistry, biology, and other branches of science that provides science information and is your ultimate destination for comprehensive study notes for high school, undergraduate, and graduate students. This Science Info domain was registered on 2002-09-19, and content has been uploaded since 2024-01-03.
Choose Notes Categories
Latest Science Notes
- Are bananas radioactive?
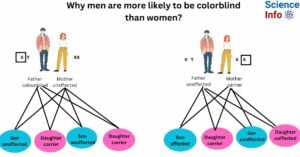
- Why men are more likely to be colorblind than women?
- Top 10 poorest countries in the world 2024
- Difference Between Alligator and Crocodile
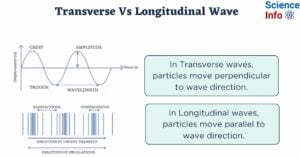
- Difference Between Transverse and Longitudinal Wave
- Gotu Kola (Centella asiatica): Amazing Medicinal Plant
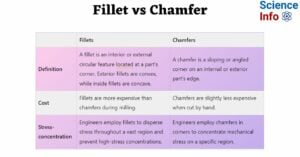
- Fillet vs Chamfer: Differences and Comparison
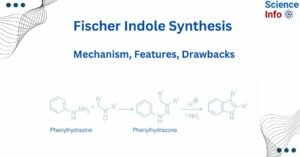
- Fischer Indole Synthesis: Mechanism, Features, Drawbacks
- Difference Between Type 1 and Type 2 Diabetes
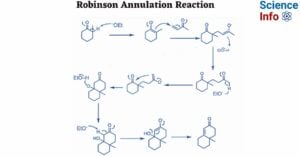
- Robinson Annulation Reaction: Mechanism, Applications
- Multimodal or Mixed-Mode Chromatography (MMC): Definition, Process, Advantages
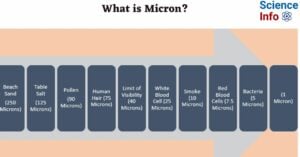
- What is Micron? How Small is Micron?
- Polycarbonate: Preparation, Structure, Properties, Applications, Advantages, Disadvantages
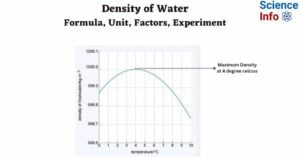
- Density of Water: Formula, Unit, Factors, Experiment
- Mylar® or BoPET: Manufacturing, Properties, Uses
- Factors affecting dye-ligand chromatography
- Coumarin Synthesis
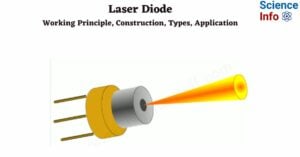
- Laser Diode: Working Principle, Construction, Types, Application
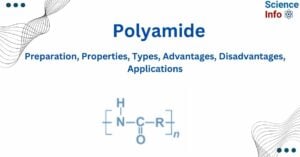
- Polyamide: Preparation, Properties, Types, Advantages, Disadvantages, Applications
- Polyamide Vs Nylon: Similarities and Differences