Amines are the derivatives of ammonia. They composed It is made up of one or more alkyl groups that replace the hydrogen atoms in an ammonia (NH3) molecule. Because of this, In amines, the nitrogen atom and the alkyl group are directly bonded. Based on the number of alkyl groups present in the compounds, amines are classified as primary secondary, and tertiary amines.
Primary amine:
Primary amines are those in which only one of the hydrogen atoms in the ammonia molecule has been changed. So the formula of the primary amine is RNH2 where “R” is an alkyl group. E.g., CH3NH2 ( methylamine)
Secondary amines
In a secondary amine, two of the hydrogens in an ammonia molecule have been replaced by alkyl groups. E.g., Dimethyl amine ( CH3)2NH2
Tertiary amines
In tertiary amines, all hydrogens in an ammonia molecule have been replaced by alkyl groups. E.g., Trimethyl amine ( CH3)3NH2
Preparation
From amide
Simple amide undergoes a reduction reaction with lithium aluminum hydride to form primary amine.
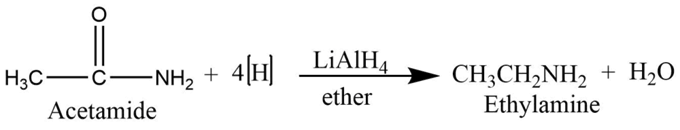
The reaction of a primary amine with an alkyl halide
Primary amine on reaction with the alkyl halide produces the dialkylammonium salt which on further reaction with NaOH yields free secondary amine.
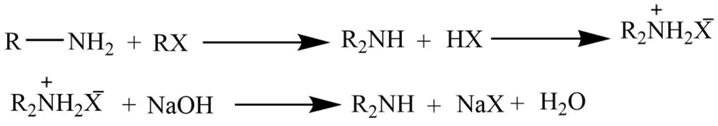
The reaction of an alkyl halide with ammonia
Tetraalkylammonium halide is formed by heating an alcoholic ammonia solution with an excess of alkyl halide. Which on reaction with NaOH solution gives free tertiary amine.
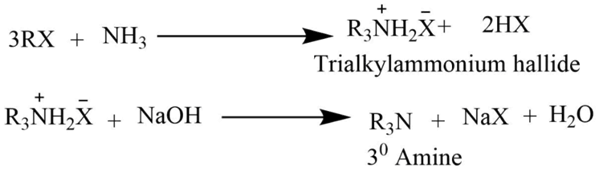
Physical properties
Boiling point
| Types of amine | Example | B.P |
| Primary amine | CH3NH2 | -6.3 0C |
| Secondary amine | (CH3)2NH | 7.4 0C |
| Tertiary amine | (CH3)3N | 3.5 |
Odor
The lower member of amine amines, such as methylamine and ethylamine, has an ammonia-like odor. As the amines grow in size, they begin to smell more “fishy” or decay-like.
Solubility
Small members of all types of amines are soluble in water. The amines which are commonly found as gases at normal temperatures are usually marketed as water solutions. All amines, including tertiary amines, can form hydrogen bonds with water.
Although tertiary amines lack hydrogen atoms and cannot form hydrogen bonds with themselves, they can form hydrogen bonds with water. Solubility decreases as the hydrocarbon chains grow longer, particularly beyond approximately Six carbons.
Basicity
The stability of the molecule generated after donating the lone pair is used to explain the basicity of amines. The nitrogen atoms gain a positive charge when it gives the lone pair of electrons. In amines, alkyl groups are directly attached to the nitrogen, So the positive charge on the nitrogen atom is decreased due to the electron-releasing property of the alkyl group. Therefore, amines are more basic than ammonia.
Primary amine
The basicity of primary amines is greater than that of ammonia but is lower than that of secondary and tertiary amines. Since primary amines contain only one alkyl group, primary amines are less basic than secondary amines.
Secondary amine
Secondary amines have higher basicity than primary and tertiary amines. The stability of the molecule generated after absorbing a proton (donating the lone pair on a nitrogen atom to a proton) is used to characterize the basicity of amines. Electron-donating groups are alkyl groups. The positive charge on the nitrogen atom in a secondary amine composed of two alkyl groups is lowered due to the electron-donating property of alkyl groups. As a result, secondary amines are more basic than other amine types.
Tertiary amine
Tertiary amines are less basic than secondary amines but more basic than primary amines and ammonia. Even though three alkyl groups decrease the positive charge on the nitrogen atom tertiary amines are less basic than secondary amines. The lower basicity of tertiary amine than that of secondary amine is due to the steric hindrance caused by three alkyl groups surrounding one nitrogen atom. As a result, the basicity of the amine is reduced. The basicity of a tertiary amine is somewhat higher or similar to that of a primary amine.
Nucleophilic addition reactions of amine (Imine and enamine formation reaction)
A chemical addition reaction in which a nucleophile establishes a sigma bond with an electron-deficient molecule is known as a nucleophilic addition reaction. These reactions are particularly significant in organic chemistry because they allow carbonyl groups to be converted into a wide range of functional groups.
Imine formation
The reaction of primary amines with aldehydes/ketones produces carbinolamines, which dehydrate to produce substituted imines. Imines are the N analogs of aldehyde and ketone O systems. Typically, the reactions are performed in an acidic buffer to activate the C=O and enable dehydration without blocking the nucleophile.
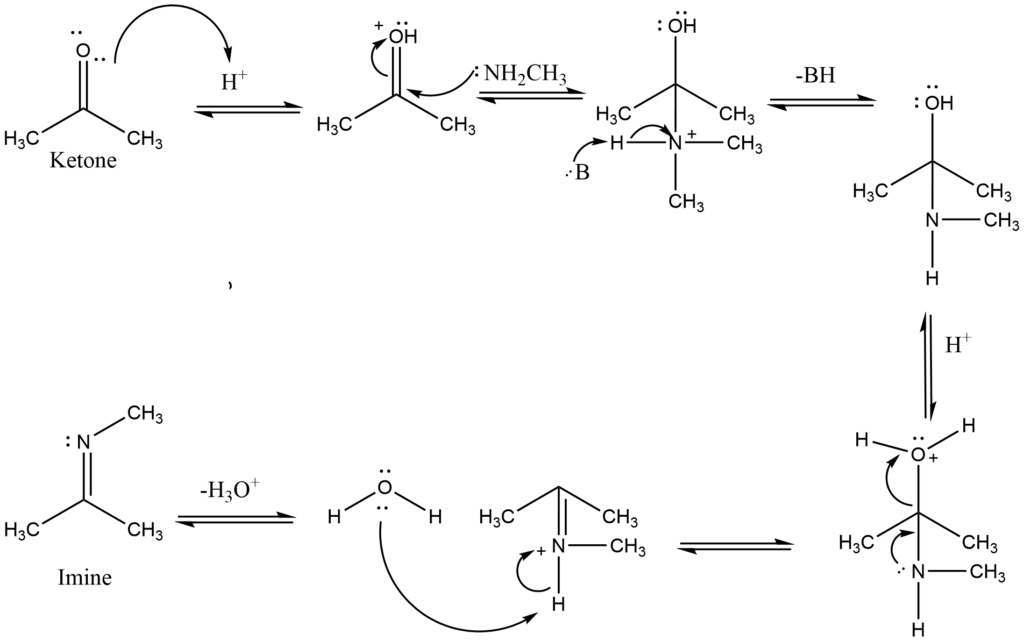
Initially, protonation of the carbonyl activates it, making it more susceptible to attack by a neutral nucleophilic, such as the N of a primary amine. The N nucleophile then attacks the electrophilic C of the C=O group, with electrons from the bond passing to the +ve O.
The removal of the proton neutralizes the +ve charge on the N, resulting in the formation of the carbinolamine intermediate. The intermediate has been protonated. The electron in N contributes to the loss of a neutral water molecule, resulting in a C=N in the form of an iminium ion. The iminium N is deprotonated, yielding the imine product and regenerating the acid catalyst.
Enamine formation
Secondary amines react with aldehydes or ketones to form carbinolamines, which dehydrate to form enamines. Since there is no N-H in carbinolamine, it can only remove to yield a C=C in these reactions. As these compounds are “alkene amines,” they are referred to as enamines.
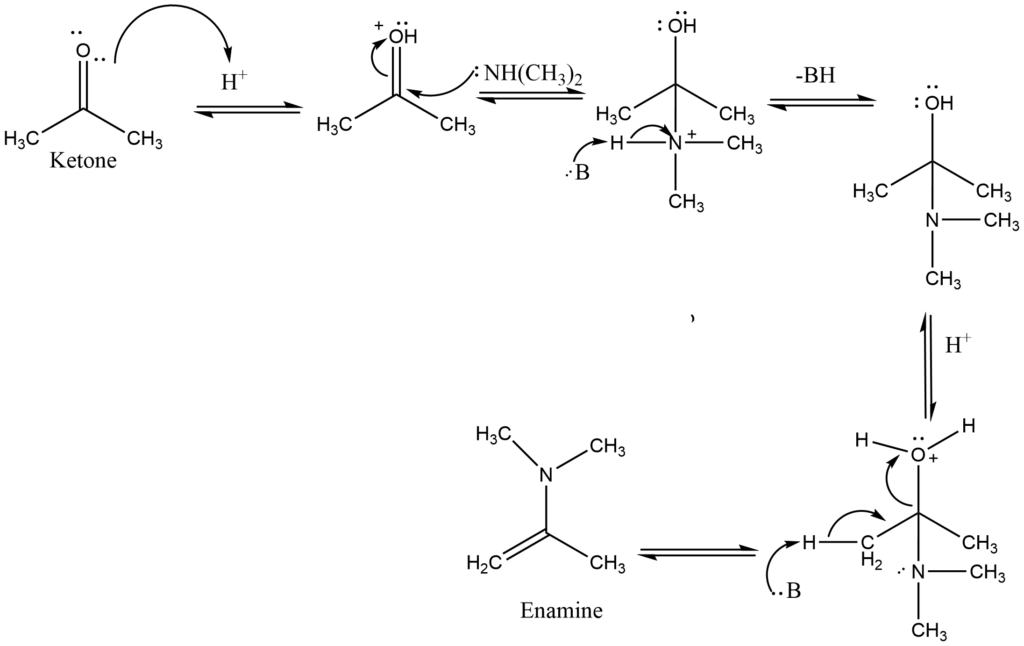
The formation of the C=C bond is enabled by the removal of a proton from a nearby C and the loss of the leaving group, a neutral water molecule results in the formation of the enamine.
Distinguishing tests for primary secondary and tertiary amines
Hinsberg test
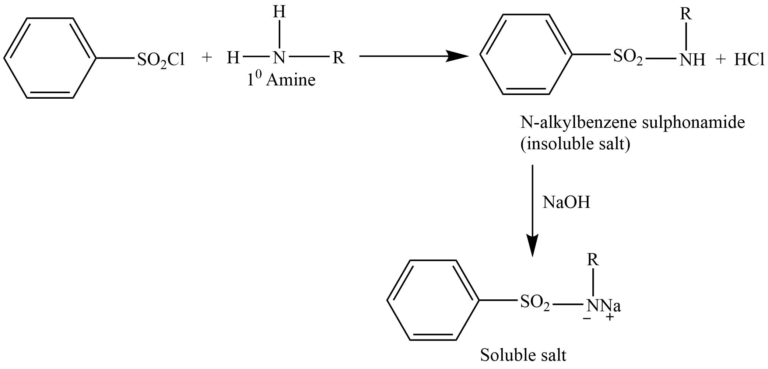
The Hinsberg test is a chemical test that is often used to identify primary, secondary, and tertiary amines. This method involves the reaction of an amine in the presence of an aqueous alkali interacting with benzenesulphonyl chloride (Hinsberg reagent).
Primary amine reacts with Hinsberg reagent to give N-alkylbenzene sulphonamide, which forms a salt with NaOH, that is soluble in water.
Secondary amine
Secondary amine reacts with Hinsberg reagent to give N, N-dialkylbenzene sulphonamide. This doesn’t react with NaOH and is insoluble in an alkaline solution.
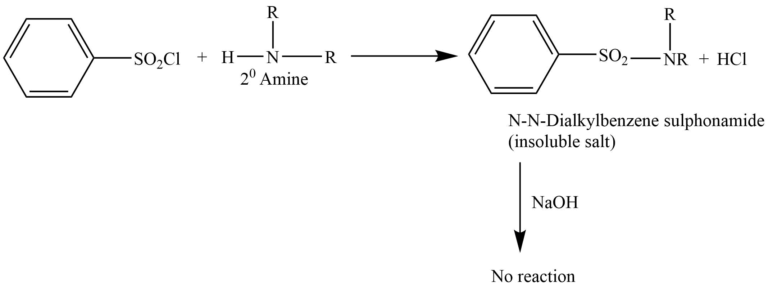
Tertiary amine does not react with the Hinsberg reagent.
Hofmann’s isocyanide test (Carbylamine reaction)
When aromatic and aliphatic primary amines are heated with chloroform and alcoholic KOH, isocyanides or carbylamines are produced, and the reaction is known as a carbylamine reaction. The newly generated substance has an unpleasant odor. The production of carbylamine or isocyanide is easily identified by its unpleasant odor.
A mixture of primary amine (aromatic or aliphatic) and chloroform is heated with alcoholic potassium hydroxide to generate alkyl isocyanide or carbylamine in the carbylamine reaction.
RNH2 + CHCl3 + 3 KOH → RNC + 3 KCl + 3H2O
The compound generated as product RNC is the alkyl isocyanide, and the reaction is known as an isocyanide test or Hoffmann’s carbylamine reaction.
Secondary and tertiary amines are not affected by the carbylamine reaction. As a result, the Hofmann isocyanide test or carbylamine reaction will not produce an unpleasant smell when used with secondary or tertiary amines.
Carbylamine reactions are exclusively effective for primary amines. As a result, it can detect the presence of primary amines.
Hofmann mustard oil reaction
The Hofmann mustard oil reaction is an organic reaction that involves heating primary amines with alcoholic carbon disulfide (CS2) and then heating them with an excess of mercuric chloride (HgCl2) to produce isothiocyanates. These isothiocyanates are responsible for the reaction’s strong odor. This smells like mustard oil.
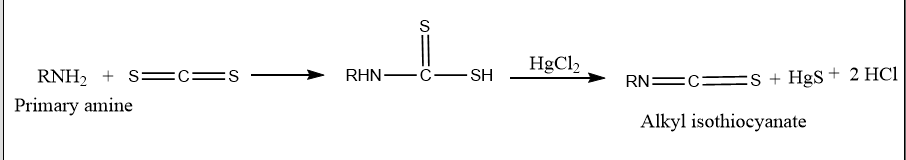
The Hofmann mustard oil reaction is used to distinguish between primary, secondary, and tertiary amines. When primary amines are treated with carbon disulfide, followed by heating with excess HgCl2, isothiocyanates are formed, which are responsible for the unpleasant odor similar to mustard oil.
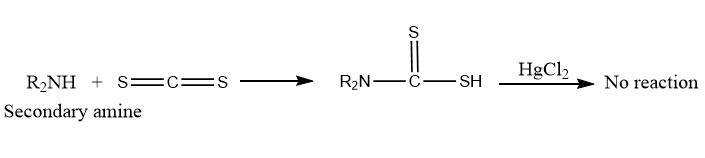
Dithiocarbamic acids are formed by the reaction of secondary amines with carbon disulfide. However, it does not react with the mercuric chloride in the following step.
Tertiary amines do not react with any of the reagents (CS2 and HgCl2).
Nitrous acid test
The reaction of primary amine with nitrous acid produces alcohol and nitrogen gas.
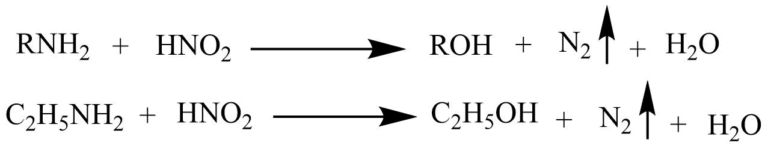
The reaction of a secondary amine with nitrous acid form N-nitrosamines that form a yellow oily layer, soluble in water.
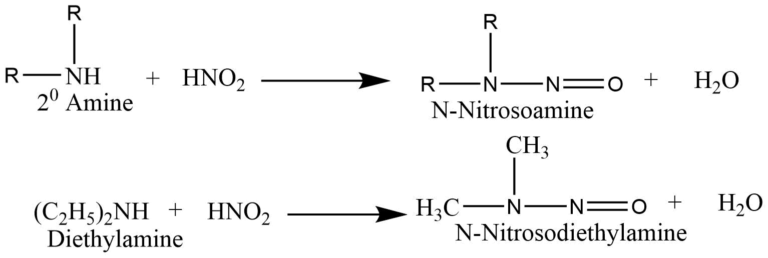
Tertiary amines react with nitrous acid to form trialkyl ammonium nitrite salts that are soluble in water, but there is no distinction in the state, color, or odor of the compound.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
- https://byjus.com/chemistry/amines-identification/.
- https://www.vedantu.com/question-answer/adistinguish-between-primary-secondary-and-class-12-chemistry-cbse-5f6584f63da50302bd590e4e.
- https://pediaa.com/difference-between-primary-secondary-and-tertiary-amines/.
