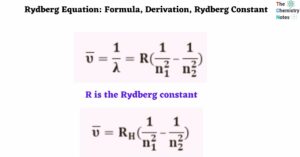
Rydberg Equation: Formula, Derivation, Rydberg Constant
The Rydberg Equation predicts the wavelength of light produced by an electron as it moves between atomic energy levels. Each element has its own spectral fingerprint. Light is produced when an … Read more