The Rydberg Equation predicts the wavelength of light produced by an electron as it
moves between atomic energy levels. Each element has its own spectral fingerprint. Light is produced when an element in its gaseous state is heated. When this light passes through a prism or a diffraction grating, brilliant lines of various hues can be seen. Each element differs from the others in some way. This discovery sparked an interest in spectroscopy.
Interesting Science Videos
What is the Rydberg Formula / Rydberg Equation?
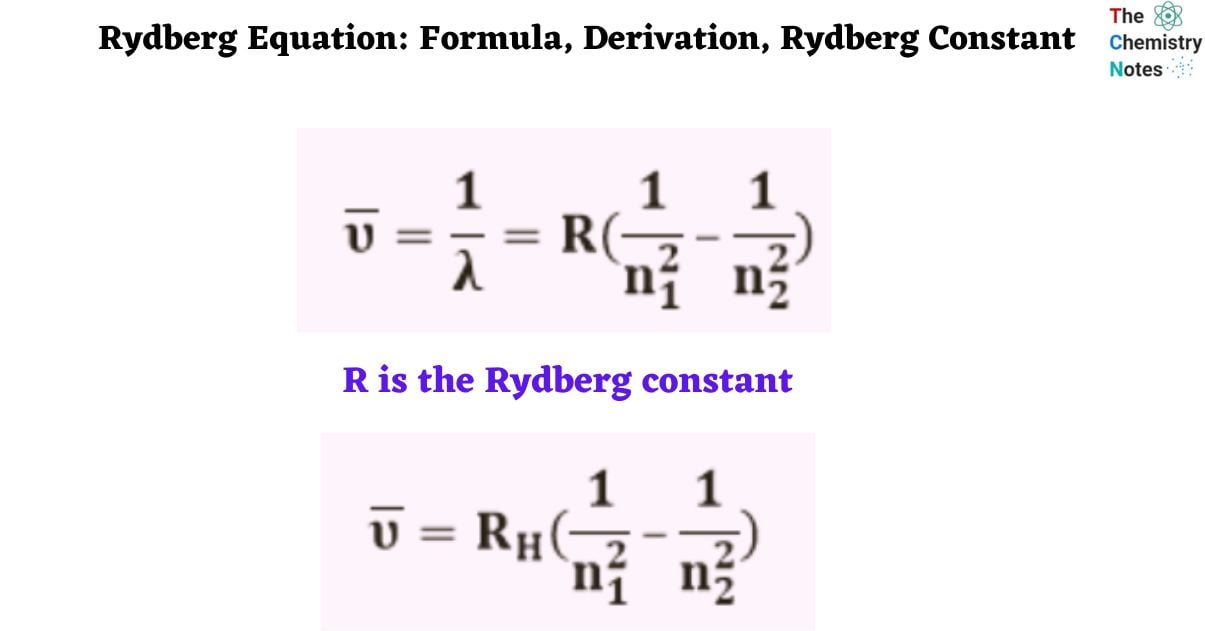
The Rydberg Equation is a mathematical method for calculating light wavelength. Each element has a unique spectral identity. The Rydberg equation can be used to evaluate elemental spectra. The wavelength of light generated by an electron moving between atomic energy levels is predicted by:
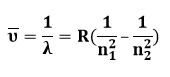
Where,
n1 and n2 are integers, and n2 is always greater than n1.
R is the Rydberg constant, and the formula is generally written as:
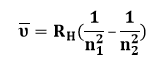
RH = Rydberg constant due to hydrogen [109677.57 cm-1]
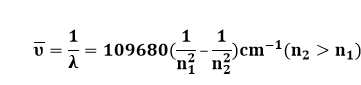
It was eventually discovered that n2 and n1 were related to the main quantum number or energy quantum number. This formula is particularly useful for transitions between energy levels of a hydrogen atom with only one electron. This formula begins to break down and produce inaccurate results for atoms with many electrons. The inaccuracy is caused by the fact that the amount of screening for inner electron or outer electron transitions varies. The equation is too simple to account for the differences.
The Rydberg formula can be used to calculate the spectral lines of hydrogen. The Lyman series is produced by setting n1 to 1 and extending n2 from 2 to infinity. Other spectral series can be determined as well:
| n1 | n2 | Converges Toward | Name |
| 1 | 2 → ∞ | 91.13 nm (ultraviolet) | Lyman series |
| 2 | 3 → ∞ | 364.51 nm (visible light) | Balmer series |
| 3 | 4 → ∞ | 820.14 nm (infrared) | Paschen series |
| 4 | 5 → ∞ | 1458.03 nm (far infrared) | Brackett series |
| 5 | 6 → ∞ | 2278.17 nm (far infrared) | Pfund series |
| 6 | 7 → ∞ | 3280.56 nm (far infrared | Humphreys series |
What is the Rydberg Constant?
The Rydberg constant defines the mathematical relationship between one element’s spectral lines and the next. When an electron moves from one orbit to another, or from a lower energy level to a higher energy level, it emits a photon of light, which is absorbed by the atom. Rydberg’s constant states that the wavelengths of successive lines in an atomic spectrum have an integer connection. The value of this constant is based on the fact that the atom which is emitting light is larger than the orbiting electron.
The Rydberg constant symbol is denoted by: RH or R∞
Application of Rydberg Constant
The Rydberg Constant is used in the following applications.
- Determines the wavelength of the hydrogen spectrum.
- Calculate the ionization energy of the transmitted electron using this formula.
- Determines their kinetic and potential energies.
Derivation of Rydberg Equation
When an electron moves from one orbit to another.
ΔE = Ef – Ei
Where,
ΔE = Energy Difference,
Ef = Final Energy,
Ei = Initial Energy.
Using Bohr’s Model,
ΔE = (-RH/nf2) – (-RH/ni2)
∴ ΔE = RH/ni2 – RH/nf2
∴ ΔE = RH(1/ni2 – 1/nf2)
∴ ΔE = 2.18 × 10-18(1/ni2 – 1/nf2)……………………. (Equation 1)
E = h
Put value of E in equation 1, we get
∴ hv = 2.18 × 10-18(1/ni2 – 1/nf2)
v = 2.18 × 10-18/h × (1/ni2 – 1/nf2) …………… (Where, h = 6.626 × 10-34)
v = 3.29 × 1015(1/ni2 – 1/nf2)…………..(Equation 2)
We have, c = λ
1/λ = v/c
Divide equation 2 by c,
v/c = 3.29 × 1015/c × (1/ni2 – 1/nf2)
= 1/λ = 1.0974 × 107(1/ni2 – 1/nf2) m-1
Video on Rydberg Equation
References
- https://byjus.com/rydberg-formula/
- https://unacademy.com/content/neet-ug/study-material/physics/rydberg-constant/
- https://www.toppr.com/guides/physics-formulas/rydberg-formula/
- https://www.geeksforgeeks.org/rydberg-formula/
- https://collegedunia.com/exams/rydberg-formula-equation-sample-questions-chemistry-articleid-2797
- Helmenstine, Todd. “What Is the Rydberg Formula and How Does It Work?” ThoughtCo, Aug. 28, 2020, thoughtco.com/what-is-the-rydberg-formula-604285.

