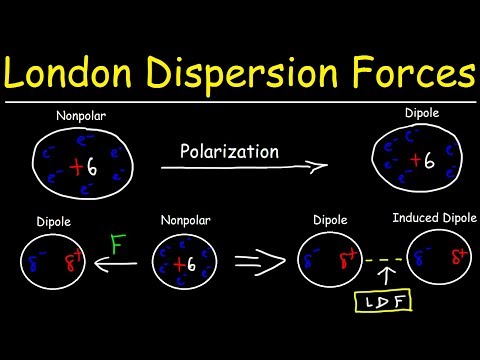
London dispersion forces are a transitory attraction between two atoms that are adjacent. The electrons of one atom are unsymmetrical, resulting in a temporary dipole. This dipole induces an induced dipole in the other atom, resulting in the attraction between the two.
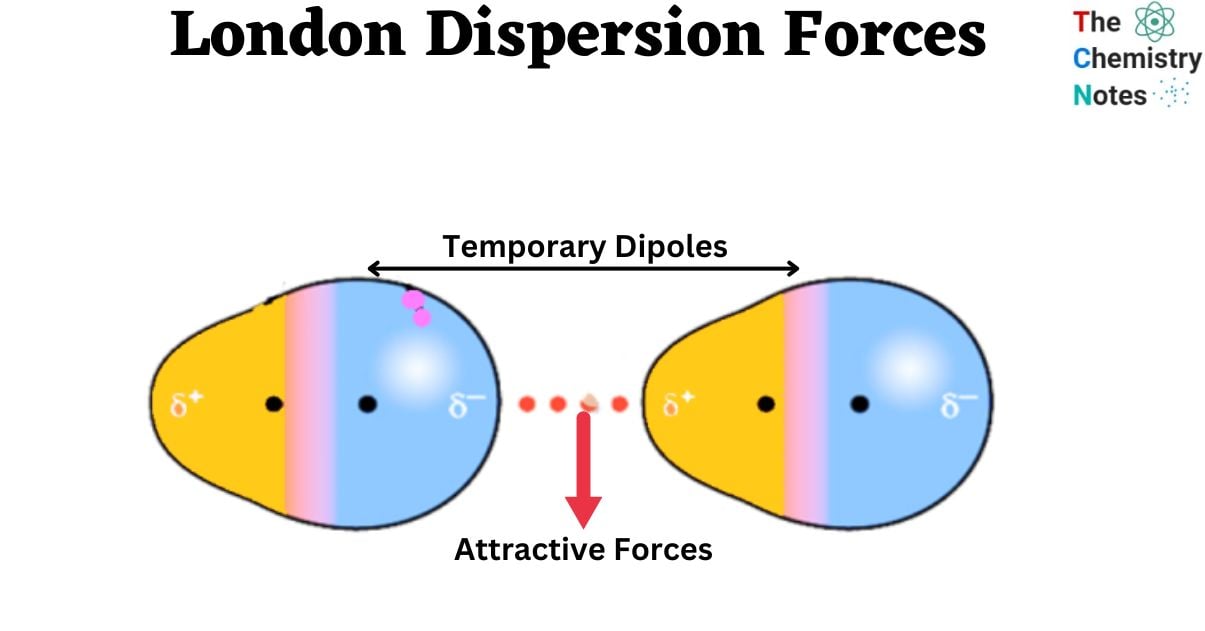
When a molecule possesses a dipole, it means that it’s electrons have an uneven distribution, resulting in a slightly positive (δ+) and slightly negative (δ-) end. Electron migration causes a transient dipole. An induced dipole is generated when a dipole forms in reaction to an adjacent dipole.
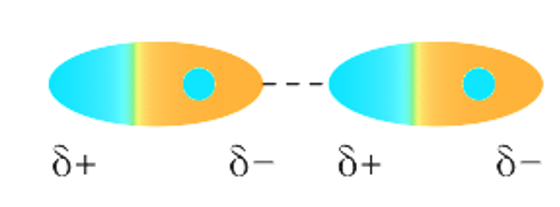
The three forms of attractive forces that occur between neutral molecules are hydrogen bonding, dipole-dipole forces, and London dispersion forces. London dispersion forces and dipole-dipole forces, in particular, are forms of intermolecular forces that are both included under the umbrella term van der Waals forces.
The order of strength of these intermolecular forces is provided below.
ion-ion > H-bonding > dipole-dipole > London’s dispersion forceWhat is London Dispersion Force?
The London dispersion force is the weakest intermolecular force. The London dispersion force is a transitory attractive force that develops when electrons in two neighboring atoms retain positions that lead the atoms to form momentary dipoles. This force is also known as an induced dipole-induced dipole attraction. London forces are the attraction forces that cause nonpolar substances to condense into liquids and freeze into solids when the temperature is sufficiently reduced.
Types of Bonds
They exist between all types of molecules, whether ionic or covalent, polar or nonpolar, and are the weakest of the intermolecular interactions. The stronger the London dispersion forces, the more electrons there are in a molecule.
Ionic Bond
A chemical bond formed when two ions with opposing charges join together. When one atom gives up one or more electrons to another, an ionic bond is formed. Salts have these sorts of bonding, which can occur between atoms or between molecules.
Covalent Bond
A coordinate covalent link, also known as a dative bond, dipolar bond, or coordinate bond, is a type of covalent connection having a specific geometry. It is a two-center, two-electron covalent bond in which both electrons come from the same atom. This type of interaction happens during the bonding of metal ions to ligands.
Properties of London Dispersion Forces
London dispersion forces have three fundamental properties:
- It is the weakest of all intermolecular forces.
- It is caused due to transient electron imbalances.
- All molecules including polar and non-polar has this force.
While these forces are modest, they are critical for non-polar molecules and noble gases. Because of these forces, they can condense into liquids or solids when the temperature drops.
Noble gases cannot become liquids without dispersion forces because no other intermolecular (between molecules or atoms) forces operate on them. Because of London dispersion forces, we may frequently utilize boiling points to predict dispersion force strength.
Molecules with strong forces will have their atoms locked together tightly, indicating that they are more likely to be in the solid or liquid phase. Atoms in a gas are bound together extremely loosely, therefore the forces between them are feeble.
Because it takes more energy to pull these atoms apart, the higher the boiling point, the greater the forces.
Factors Affecting London Dispersion Force
Three factors influence the strength of London dispersion forces:
Molecular Size
Dispersion forces exist between all molecules, whether polar or nonpolar.
- Larger and heavier atoms and molecules have larger dispersion forces than smaller and lighter ones.
- The strength of London dispersion forces is related to a molecule’s polarizability.
- The more readily polarized the forces, the stronger they are. Larger atoms and molecules are more readily polarized because their outer shell electrons are further away from the nucleus and hence bound less securely.
- This implies they are more likely to be pulled or impacted by a nearby dipole.
- The term “polarizability” refers to how easily the electron distribution around an atom or molecule may be altered.
- At room temperature, for example, Cl2 is a gas, whereas Br2 is a liquid, because the stronger forces allow bromine to be a liquid, whereas they are too weak in chlorine.
Molecular Shape
The magnitude of the dispersion forces between molecules is additionally affected by the shapes of the molecules.
- For example, Neopentane (C5H12) and n-pentane (C5H12) are both gases at ambient temperature.
- Despite the fact that both molecules are nonpolar and have the same molecular weight, the London dispersion forces between n-pentane molecules are greater than those between neopentane molecules.
- The relatively cylindrical form of n-pentane molecules permits them to interact with one other more efficiently than the relatively spherical neopentane molecules.
Molecular Distance
- Because distance is a factor (far distant = weaker), how readily molecules may approach near to one other influences strength.
- The number of “points-of-contact” influences the difference in isomer London dispersion force strengths.
Formula of London Dispersion Forces
Polarizability refers to a molecule’s propensity to create induced dipoles.
The symbol μ represents the strength of the interaction between two dipoles. The strength of the electric field is precisely related to the force (E).
μ = α × EHere,
- μ represents induced dipole moment
- α represents polarizability
- E represents the electric field.
The London dispersion force formula may be used to compute the interaction energy.
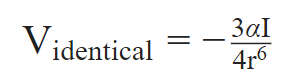
This formula calculates the potential energy between two identical atoms or molecules. The formula was updated by German scientist Fritz London for two non-identical atoms or molecules, as shown below.
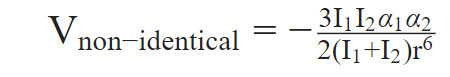
Here,
- I = Ionization energy
- Α = Polarizability
- r = Distance between molecules
Examples of London Dispersion Forces
London dispersion forces are present in nonpolar molecules. Here are a few examples:
- The noble gases are krypton (Kr), fluorine(F2), chlorine, bromine (Br), and Iodine (I). Other gases include helium (He), neon (Ne), argon (Ar), and neon (Ne).
- For example, the only interactions that exist between iodine (I2) molecules are the London dispersion forces, which are relatively weak. Because the intensity of these forces is consistent with iodine’s enormous electron cloud and polarizability, it exists as a solid at room temperature.
- For one helium atom, London dispersion forces induce a dipole to develop on an adjacent helium atom. Since helium is a noble gas, it has the lowest boiling point because the only intermolecular interactions present are dispersion forces, which are the weakest.
Consequences of London Dispersion Forces
Because polarizability impacts how quickly atoms and molecules form connections with one another, it also affects attributes like as melting and boiling points. For example, because they are both halogens, you could predict Cl2 (chlorine) and Br2 (bromine) to behave similarly. But at normal temperature, bromine is a liquid, whereas chlorine is a gas. The bigger bromine atoms are brought together by London dispersion forces to form a liquid, but the smaller chlorine atoms have sufficient energy to keep the molecule gaseous.
Frequently Asked Questions (FAQ)
Why are London forces known as dispersion forces?
London coined the name “dispersion effect” since his theory and the quantum mechanical theory of light dispersion are fairly comparable. In physics, the term “dispersion” refers to a quantity’s variation with frequency, in this case the fluctuation of electrons in the case of the London dispersion.
What causes the London dispersion forces?
The London dispersion forces are produced by the Coulombic attraction between the dipoles.
What is the importance of London dispersion forces?
London Dispersion Forces, while being regarded as the weakest intermolecular force, are crucial in larger molecules. Due to the absence of functional groups, it is employed to bind the non-polar molecule.
Why do London dispersion forces rise as molecular size increases?
The atomic or molecular weight of the substance is often what determines the London dispersion forces. There are more electrons and stronger London forces in heavier atoms and molecules. Thus, melting or boiling them is more challenging. This discusses the molecular states of halogens at room temperature.
Video on London Dispersion Forces
References
- https://www.chem.purdue.edu/gchelp/liquids/disperse.html#:~:text=The%20London%20dispersion%20force%20is,induced%20dipole%2Dinduced%20dipole%20attraction.
- https://byjus.com/chemistry/london-dispersion-forces/
- https://chem.libretexts.org/Bookshelves/General_Chemistry/General_Chemistry_Supplement_(Eames)/Phases_and_Intermolecular_Forces/London_Dispersion_Forces
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/london-dispersion-forces/
- https://unacademy.com/content/neet-ug/study-material/chemistry/dispersion-forces-or-london-forces-definition-examples-types-and-formula/
- https://www.vedantu.com/chemistry/london-dispersion-forces
- https://www.chegg.com/learn/topic/london-dispersion-forces