A strong acid is one that is completely dissociated in water. H+ is a chemical species with a high potential for proton loss. A molecule with a hydrogen bonded to an electronegative atom, such as oxygen or a halogen (fluorine, chlorine, bromine, iodine, astatine, and tennessine), is classified as a strong proton donor. Strong acids react with water by losing a proton and giving it to the H2O molecules, which results in the formation of H3O, or a hydronium ion:
HA (aq) + H2O → H3O+ (aq) + A− (aq)
Although diprotic and polyprotic acids can lose more than one proton, the “strong acid” pKa value and reaction only pertain to the loss of the first proton.
These reactions are always reversible, but in certain circumstances, the acid is so excellent at giving away hydrogen ions that we may conceive of the reaction as one-way. The acid is nearly completely ionized.
Interesting Science Videos
Strong Acids pH and pKa
pH is a measure of the concentration of hydrogen ions in a solution. Strong acids, such as hydrochloric acid, have a pH range of 0 to 1. The concentration of hydrogen ions in the solution increases as the pH decreases.
pH = -log [H+]The acid dissociation constant, Ka, quantifies how fully an acid dissociates in aqueous solution. A high Ka value suggests a strong acid since it signifies the acid has been substantially dissociated into its ions. Strong acids have a low Ka value because they totally dissociate in solution.
The logarithmic acid dissociation constant, pKa, has a direct connection with Ka. As a result, the lower the pKa value, the stronger the acid.
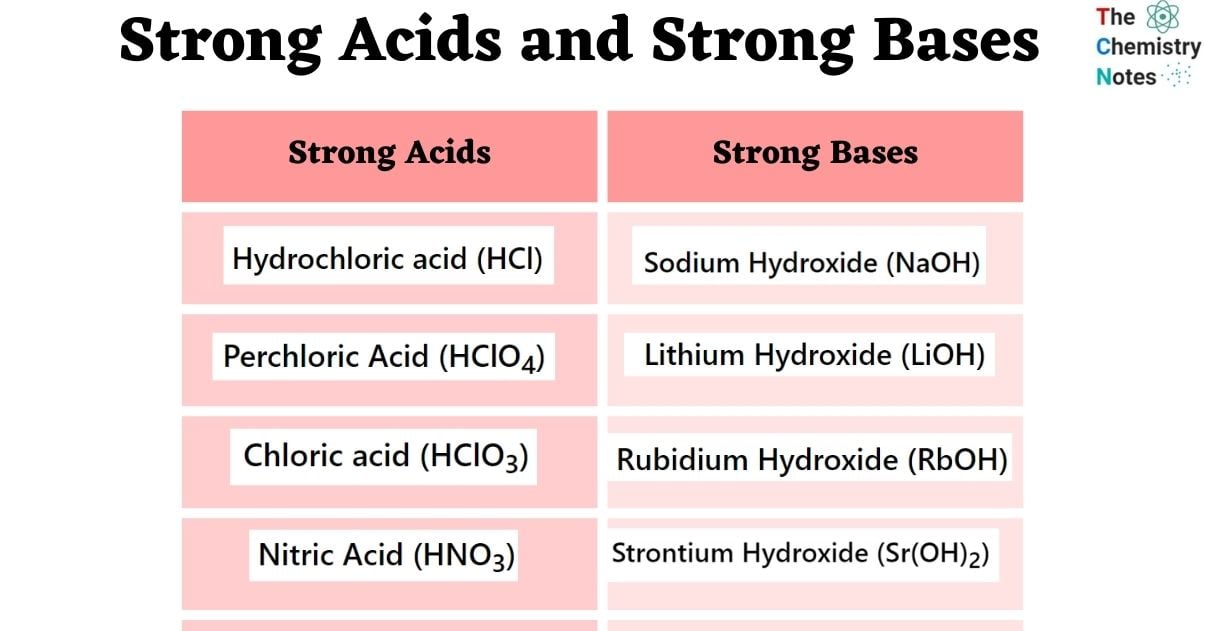
pKa = -log [Ka]Seven acids are usually recognized as “strong” acids in the area of chemistry. A list of strong acids is shown below.
- Hydrochloric Acid (HCl)
- Hydrobromic Acid (HBr)
- Hydroiodic Acid or Hydriodic Acid (HI)
- Sulfuric Acid (H2SO4)
- Nitric Acid (HNO3)
- Chloric Acid (HClO3)
- Perchloric Acid (HClO4)
Types of Strong Acids
Strong acids are discussed briefly here:
Hydrochloric acid (HCl)
- Hydrochloric acid, often known as muriatic acid, is a chemical compound having the formula HCl. This is a colorless inorganic acid.
- This inorganic acid is highly acidic and has a strong odor. It should be handled with caution since it might cause skin irritation upon contact.
- This corrosive acid also has the simplest acidic system (it comprises chlorine and water). It is just a solution of hydrogen chloride in water.
- The chloride ion and the hydronium ion are two chemical constituents in this system. Furthermore, this is one of those acids that is very important in biology.
- HCl is a natural component of gastric acid (the acid that forms spontaneously in the digestive systems of practically all mammals). In fact, the human digestive system naturally creates it since it assists in food digestion.
- Molar Mass: 36.46 g/mol
- Density: 1.19 g/cm3
- Boiling Point: Depends on the concentration
- Melting Point: Depends on the concentration
- Acidity pKa: −5.9 (HCl gas)
Perchloric Acid (HClO4)
- Perchloric acid, having the chemical formula HClO4, is an extremely potent mineral acid. This chemical is colorless and is often employed in aqueous solutions.
- Perchloric acid is a more powerful acid than sulfuric acid and nitric acid. This chemical is an extremely effective oxidant when kept dry.
- Because of the acid’s high oxidizing capabilities, there are various rigorous requirements that must be followed when handling it.
- This is especially true when the acid comes into touch with metals such as aluminum (Al), organic materials such as wood, and some types of polymers.
- This is due to the acid’s strong reactivity in its presence. In fact, all research on this powerful acid must be conducted in fume hoods.
- Molar Mass: 100.46 g/mol
- Density: 1.768 g/cm3
- Boiling Point: 203°C (397°F) 476 K
- Melting Point: −17°C (1°F) 256 K
- Acidity pKa: −15.2 (±2.0)
- Conjugate Base: Perchlorate
Hydroiodic Acid or Hydriodic Acid (HI)
- Hydroiodic acid (also known as hydriodic acid) is a very acidic solution of hydrogen iodide and water.
- This chemical is reported to be the second strongest hydrogen and halogen acid (the strongest being hydrostatic acid).
- Hydroiodic acid is an extensively used chemical reagent. When introduced in an aqueous solution, this chemical can totally ionize.
- Under typical temperature and pressure circumstances, this chemical exists as a colorless liquid with a mildly pungent odor.
- Molar Mass: 127.91 g/mol
- Density: 1.70 g/mL
- Boiling Point: 127°C (261°F 400 K)
- Acidity pKa: -9.3
- Conjugate Base: Iodonium
Chloric acid (HClO3)
- The chemical compound chloric acid is an oxoacid of chlorine with the formula HClO3.
- This chlorine oxoacid is widely used as a potent oxidizing agent and is very acidic in nature.
- Chloric acid is thermodynamically unstable. The reason for this is because this chemical molecule is easily disproportionate.
- Under typical circumstances, this acid oxidizes and reduces concurrently, yielding two distinct end products (H+ and an anion).
- There is, however, a technique to make this acid more stable. This can happen in chilly conditions or when immersed in an aqueous solution with a concentration of no more than 30%.
- Molar Mass: 84.45914 g/mol
- Density: 1 g/mL
- Boiling Point: Depends on the concentration
- Melting Point: Depends on the concentration
- Acidity pKa: −2.7
- Conjugate Base: Chlorate
Hydrobromic Acid (HBr)
- When hydrogen bromide, a diatomic molecule made up of one hydrogen and one bromine atom, is dissolved in water, a highly solid acid called hydrobromic acid is created.
- Hydrobromic acid is a more potent acid than hydrochloric acid. However, when it comes to acidic strength, this acid is not as strong as hydroiodic acid.
- Hydrobromic acid is largely employed in the synthesis of inorganic bromides, particularly calcium, zinc, and sodium bromides.
- This chemical is an excellent reagent for the synthesis of organ bromine compounds.
- HBr is used to cleave many ethers.
- This chemical also catalyzes certain alkylation processes, which are useful in mineral extraction.
- Molar Mass: 80.91 g·mol−1
- Density: 1.49 g/cm3
- Boiling Point: 122°C (252°F) 395 K at 700 mmHg
- Melting Point: −11°C (12°F) 262 K
- Acidity pKa: −9
- Conjugate Base: Bromide
Nitric Acid (HNO3)
- Nitric acid (chemical formula HNO3), often known as the spirit of nitre and occasionally as aqua fortes, is a very corrosive mineral acid.
- This chemical is a strong oxidizer and is commonly employed in nitration processes that require the addition of a nitro group to a given reactant.
- It occurs in liquid form under standard temperature and pressure (STP) conditions.
- Apart from that, it might be colorless, yellowish-red, or yellow under STP.
- Additionally, the stench is quite strong, stifling, and unpleasant.
- Molar Mass: 63.012 g·mol−1
- Density: 1.51 g/cm3
- Boiling Point: 83°C (181°F) 356 K
- Melting Point: −42°C (−44°F) 231 K
- Acidity pKa: −1.4
- Conjugate Base: Nitrate
Sulfuric acid (H2SO4)
- Sulfuric acid (also known as vitriol oil or oil of vitriol) is a mineral acid composed of oxygen, sulfur, and hydrogen.
- This acid is a colorless, odorless, and very viscous liquid. It should also be mentioned that sulfuric acid is water soluble and is typically generated in highly exothermic processes.
- The corrosive behavior of this substance can be traced mostly to its strong acidic nature (as well as its oxidizing capabilities when stored at high concentrations).
- It is also well known that sulfuric acid is hygroscopic. It quickly takes up airborne water vapor.
- It is essential to remember that sulfuric acid can result in severe chemical burns and even subsequent thermal burns. Therefore it is considered to be extremely harmful even at lower quantities.
- Molar Mass: 98.079 g/mol
- Density: 1.8302 g/cm3
- Boiling Point: 337°C (639°F) 610 K
- Melting Point: 10.31°C (50.56 °F) 283.46 K
- Acidity pKa: pKa1 = −2.8
pKa2 = 1.99 - Conjugate Base: Bisulfate
Conjugate Base of Strong Acid
- The conjugate base of an acid is the ion formed when the acid loses a proton.
- Salts derived from such conjugate bases frequently have a pH of 7 or slightly higher.
- Sodium chloride, sodium sulfate, and sodium nitrate, for example, all contain conjugate bases of strong acids as anions.
- All of those salts have a pH approaching 7 in solution, which is a neutral pH.
Strong Base
A strong base (BOH) also totally dissociates and completely ionizes in an aqueous solution. Furthermore, strong bases are good proton acceptors, which cannot exist in aqueous solution. For instance, all O2- ions are transformed to OH–, hydroxide ions, by absorbing protons from H2O molecules. As a result, H2O molecules are transformed to OH–. The cations of strong bases are soluble in water. In other words, if a cation is soluble in water, it can generate a strong base.
BOH + H2O → B+ (aq) + OH– (aq)
A weak base, on the other hand, only partially dissociates into its ions in water. A excellent example of a weak base is ammonia.
Strong bases react with strong acids to generate stable molecules.
Solid bases have high pH close to 14 and can be extremely damaging and dangerous in excessive concentrations. Solid bases, like all bases, have an unusual texture and a foamy flavor. Regardless, it is never a good idea to taste a solid base because of its appealing aspect.
Strong Bases pOH and pKb
The concentration of hydroxide ions (OH–) is measured by the pOH. The lower the pOH value, the more hydroxide ions are present in the solution, and hence the stronger the base.
pOH = -log [OH–]The base dissociation constant, Kb, quantifies how fully a base dissociates in aqueous solution. A high Kb value suggests a strong base since it signifies the base has been substantially dissociated into its ions.
With the Kb value, you can compute pKb. The lower the pKb number, the more stable the base.
pKb = -log [Kb]The pH and pOH of an aqueous solution are connected by the following equation:
pH + pOH = 14If the pH or pOH value of a solution is known, the other can be computed. The pH of strong bases is typically between 13 and 14.
Eight bases are usually recognized as “strong” bases in the area of chemistry. A list of strong bases is shown below.
- Sodium Hydroxide (NaOH)
- Potassium Hydroxide (KOH)
- Lithium Hydroxide (LiOH)
- Rubidium Hydroxide (RbOH)
- Cesium Hydroxide (CsOH)
- Calcium Hydroxide (Ca(OH)2)
- Barium Hydroxide (Ba(OH)2)
- Strontium Hydroxide (Sr(OH)2)
Sodium Hydroxide (NaOH)
- Sodium hydroxide, widely known as lye and caustic soda, is an inorganic chemical having the formula NaOH.
- It is a white, solid ionic compound composed of sodium cations (Na+) and hydroxide anions (OH).
- At room temperature, sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins and can inflict serious chemical burns.
- It collects moisture and carbon dioxide from the air and is extremely soluble in water.
- Sodium hydroxide is utilized in a variety of sectors, including the manufacture of wood pulp and paper, textiles, drinking water, soaps and detergents, and as a drain opener.
- Molar Mass: 39.9971 g/mol
- Density: 2.13 g/cm3
- Melting Point: 323°C (613°F) 596 K
- Boiling Point: 1388°C (2530°F) 1661 K
- Acidity pKa: 15.7
Potassium Hydroxide (KOH)
- Potassium hydroxide, often known as caustic potash, is an inorganic compound having the formula KOH.
- KOH, like sodium hydroxide (NaOH), is a model strong base.
- KOH has excellent thermal stability. Because of its excellent stability and low melting point, it is frequently melt-cast into pellets or rods, which have a low surface area and are easy to handle.
- Because of its corrosive qualities, potassium hydroxide is a helpful element in agents and preparations that clean and disinfect surfaces, as well as materials that can withstand corrosion by KOH.
- Molar Mass: 56.11 g mol−1
- Density: 2.044 g/cm3 (20 °C)
- Melting Point: 360°C (680°F) 633 K
- Boiling Point: 1327°C (2421°F) 1600 K
- Acidity pKa: 14.7
Lithium Hydroxide (LiOH)
- Lithium hydroxide LiOH, is an inorganic chemical.
- It exists as an anhydrous or hydrated solid, both of which are white hygroscopic solids.
- They are water soluble and mildly soluble in ethanol. Both are commercially accessible.
- Despite being categorized as a strong base, lithium hydroxide is the weakest alkali metal hydroxide known.
- Lithium hydroxide is primarily used in the manufacturing of lithium ion battery cathode materials such as lithium cobalt oxide (LiCoO2) and lithium iron phosphate.
- Molar Mass: 23.95 g/mol (anhydrous) 41.96 g/mol (monohydrate)
- Density: 1.46 g/cm3 (anhydrous) 1.51 g/cm3 (monohydrate)
- Melting Point: 462°C (864°F) 735 K
- Boiling Point: 924°C (1695°F) 1197 K (decomposes)
- Acidity pKa: 14.4
Rubidium Hydroxide (RbOH)
- The inorganic chemical with the formula RbOH is called rubidium hydroxide.
- It is made up of rubidium cations and an equal amount of hydroxide anions.
- It is a colorless solid that is commercially accessible in aqueous solutions from a few sources.
- Rubidium hydroxide, like other strong bases, is very corrosive.
- Rubidium hydroxide is generated when rubidium metal combines with water.
- Molar Mass: 102.475 g·mol−1
- Density: 3.1 g/mL at 25 °C
- Melting Point: 382°C (720°F) 655 K (decomposes)
- Boiling Point: 1390°C (2530°F) 1660 K
- Acidity pKa: 15.4
Cesium Hydroxide (CsOH)
- Cesium hydroxide, like other alkali metal hydroxides such as sodium and potassium hydroxides, is a strong base containing the extremely reactive alkali element cesium.
- It is the most powerful of the five alkali metal hydroxides.
- Because of its strong reactivity, cesium hydroxide is particularly hygroscopic.
- Cesium hydroxide in laboratories is usually a hydrate.
- Because cesium is expensive to extract and has a reactive nature, this substance is not frequently employed.
- Molar Mass: 149.912 g/mol
- Density: 3.675 g/cm3
- Melting Point: 272°C (522°F) 545 K
- Solubility In Water: 300 g/100 mL at 30 °C
- Acidity pKa: 15.76
Calcium Hydroxide (Ca(OH)2)
- Calcium hydroxide (also known as slaked lime) is an inorganic substance having the formula Ca(OH)2.
- It is a colorless crystal or white powder that is formed when quicklime (calcium oxide) is combined with water.
- It is also known as hydrated lime, caustic lime, builders’ lime, slaked lime, cal, and pickling lime.
- It is also used in fresh-water treatment to raise the pH of the water so that pipes do not corrode when the base water is acidic, because it is self-regulating and does not raise the pH too high.
- Molar Mass: 74.093 g/mol
- Density: 2.211 g/cm3
- Melting Point: 580°C (1076°F) 853 K (loses water, decomposes)
- Solubility In Water: 1.89 g/L (0°C); 1.73 g/L (20°C); 0.66 g/L (100°C)
- Acidity pKa: 12.63 (first OH−), 11.57 (second OH−)
Barium Hydroxide (Ba(OH)2)
- Barium hydroxide is a chemical compound having the formula Ba(OH)2.
Barium hydroxide may be made by dissolving barium oxide (BaO) in water. - Barium hydroxide is utilized as a precursor to various barium compounds in industry.
In analytical chemistry, barium hydroxide is used to titrate weak acids, particularly organic acids. - Barium hydroxide, like other strong bases and water-soluble barium compounds, is caustic and poisonous, causing skin irritation and burns as well as eye damage.
- Molar Mass: 171.34 g/mol (anhydrous); 189.355 g/mol (monohydrate)
- Density: 3.743 g/cm3 (monohydrate)
- Melting Point: 407 °C (anhydrous); 300 °C (monohydrate)
- Solubility In Water: 1.67 g/100 mL (0°C); 3.89 g/100 mL (20°C); 4.68 g/100 mL (25°C)
- Basicity pKb: 0.15 (first OH–); 0.64 (second OH–)
Strontium Hydroxide (Sr(OH)2)
- Sr(OH)2 is a caustic alkali that contains one strontium ion and two hydroxide ions. It is made by mixing a strontium salt and a strong base.
- Sr(OH)2 can be anhydrous, monohydrate, or octahydrate.
- Because Sr(OH)2 is only marginally soluble in cold water, it may be easily prepared by adding a strong base, such as NaOH or KOH, drop by drop to a solution of any soluble strontium salt, most frequently Sr(NO3)2 (strontium nitrate).
- Strontium hydroxide is primarily employed in the refining of beet sugar and as a plastic stabilizer.
Video on Strong Acids and Strong Bases
References
- https://en.wikipedia.org/wiki/Strontium_hydroxide
- https://en.wikipedia.org/wiki/Barium_hydroxide
- https://en.wikipedia.org/wiki/Calcium_hydroxide
- https://byjus.com/chemistry/examples-of-bases/
- https://unacademy.com/content/jee/study-material/chemistry/strong-acid-and-strong-base/
- https://www.thoughtco.com/definition-of-strong-base-604664
- https://www.turito.com/blog/one-on-one-online-tutoring/list-of-top-strong-acids
- https://www.chemguide.co.uk/physical/acidbaseeqia/acids.html
- https://chemistrytalk.org/strong-acids-bases/
- https://en.wikipedia.org/wiki/Hydrogen_iodide
- https://byjus.com/chemistry/list-of-strong-acids/

