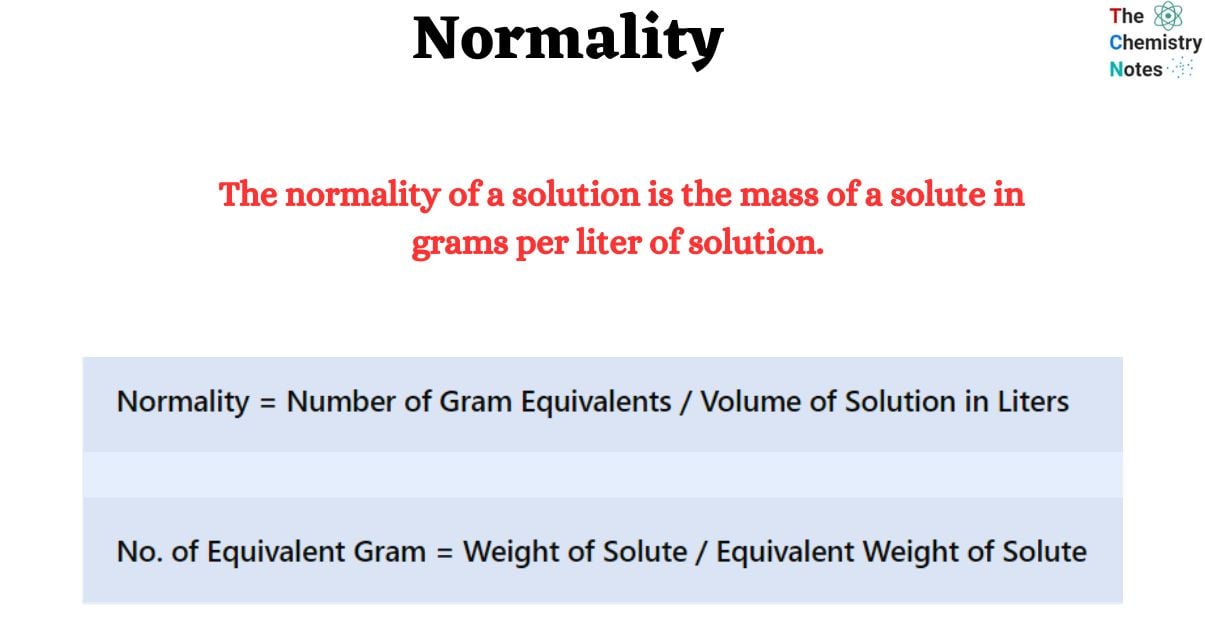
In chemistry, normality is one of the terms used when referring to the concentration of a solution. The normality of a solution is the mass of a solute in grams per liter of solution. It is sometimes referred to as the comparable concentration. The sign ‘N’ symbolizes units of concentration. Symbols such as eq L-1, or meq L-1 (= 0.001 N) are also used for units of concentration. It is mainly used for evaluating reactive chemicals in solution, during titration reactions, or in conditions requiring acid-base chemistry.
Interesting Science Videos
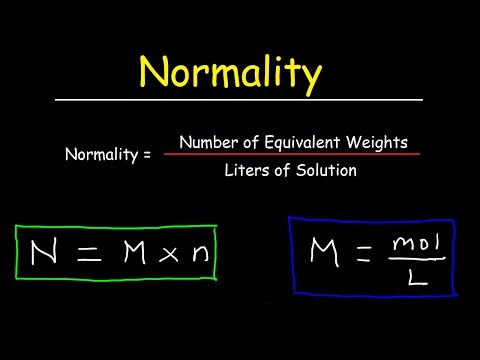
Normality Formula
The normality equation is applied to calculate the concentration of a given solution. When all of the steps are accurately carried out, the formula for normality is established. The formula is as follows:
Normality = Number of Gram Equivalents / Volume of Solution in LitersNo. of Equivalent Gram = Weight of Solute / Equivalent Weight of SoluteTherefore,
N = Weight of Solute in gm. / Equivalent Weight × Volume in litreThe solution’s molarity value may be used to calculate the normality. Consequently, the formula will be expressed as:
Normality (N) = Molarity × Molar mass / Equivalent massNormality (N) = Molarity × Basicity = Molarity × AcidityUses of Normality
Normality is most commonly used in three situations:
- For determining concentrations in acid-base chemistry. In acid-base chemistry, normalcy is used to determine the concentrations of hydronium (H3O+) and hydroxide (OH–). In this scenario, the fraction 1/feq is a positive integer.
- In precipitation reactions, it is used to calculate the number of ions that are expected to precipitate in a particular reaction. In this scenario, the fraction 1/feq is a positive integer.
- It is used in redox processes to calculate the amount of electrons that a reducing or oxidizing substance may receive or donate. In this scenario, the fraction 1/feq is a positive integer.
Steps to Calculate Normality
When computing equivalents or component weights, it is critical to determine how many equivalents have been generated. Typically, it’s necessary to examine the valence, molecular mass, and whether a substance dissociates or dissolves fully.
- Collecting information regarding the equivalent weight of the reactive species contained in the solution is an initial stage.
- After that, when the equivalent weight of the species is obtained, compute the number of grams equivalent to the solute.
- After you’ve calculated both numbers, use the normality formula to compute the substance’s volume in liters.
- Finally, normality is determined by using a formula and substituting values.
Calculation of Normality in Titration
Titration is the process of gradually adding a solution of a certain volume and concentration to another solution of unknown concentration until the reaction is neutralized. To determine the normality of the acid and base titrations, use the following formula:
N1 V1 = N2 V2
Where,
N1 = Normality of the Acidic Solution
V1 = volume of the acidic solution
N2 = normality of the basic solution
V3 = volume of the basic solutionNormality Equation
Before we can compute the normality equation, let’s first understand what a mixture is considered normal.
Assuming Na and Nb normality and Va and Vb volumes:
Where,
- Na = Normality of the acidic solution
- Va = Volume of the acidic solution
- Nb = Normality of the basic solution
- Vb = Volume of the basic solution
These two solutions are combined to create a combination whose Volume is Va + Vb and whose Normality takes into account N.
Consequently, the following represents the mixture’s Normality Formula:
N = (Na Va + Nb Vb) / (Va + Vb)If four solutions with the same solute of normality and volume are combined, the resulting normality is given by,
NR = [ Na Va + Nb Vb + Nc Vc + Nd Vd ] × [ Va + Vb + Vc + Vd ]-1When four solutions (na, nb, nc, and nd) comprised of different solutes of molarity, volume, and H+ ions are combined, the resulting normalcy is given by,
NR = [naMaVa + nbMbVb + ncMcVc + ndMdVd] × [Va+Vb+Vc+Vd]-1Limitations of Normality
A lot of researchers employ normality in acid-base chemistry to avoid calculating mole ratios or just to obtain more precise outcomes. While normalcy is often used in precipitation and redox processes, it has some limitations. Limitations of normality are as follows:
- There must be a known equivalence factor for normality.
- A chemical solution’s normality is not a fixed value. Its value differs based on the chemical process being studied. A solution of CaCl2 that is 2 N with regard to the chloride (Cl–) ion, for example, is only 1 N with respect to the magnesium (Mg2+) ion.
Video Reference
References
- https://www.dentonisd.org/cms/lib/tx21000245/centricity/domain/1192/molarity.pdf
- https://byjus.com/jee/normality/
- https://www.vedantu.com/iit-jee/normality
- https://unacademy.com/content/nda/study-material/chemistry/normality-and-its-formula/
- https://www.toppr.com/guides/chemistry/solutions/normality-formula-definition-and-examples/
- https://www.thoughtco.com/how-to-calculate-normality-609580
- https://collegedunia.com/exams/normality-formula-calculation-molarity-examples-chemistry-articleid-5785