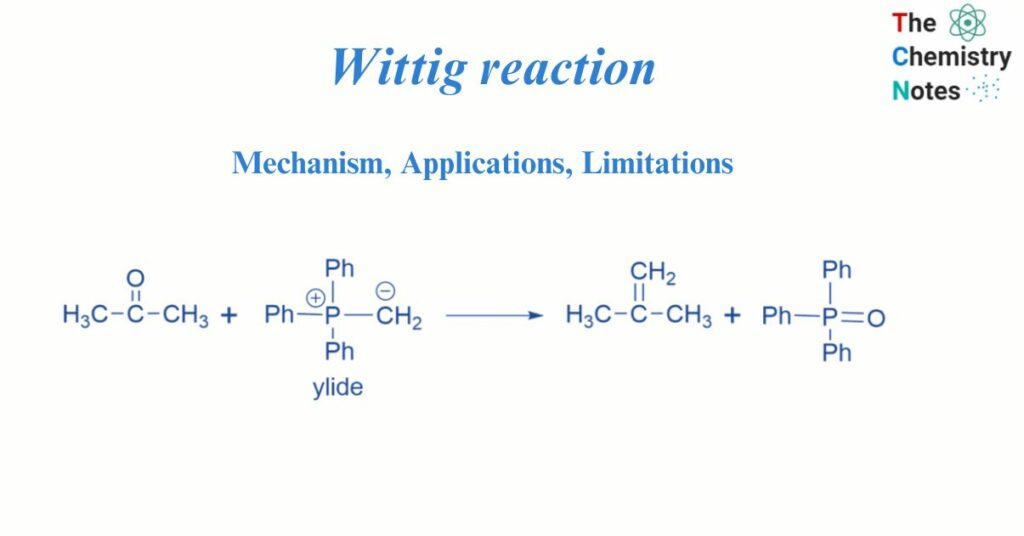
Wittig reaction facilitates the formation of an alkene by reacting an aldehyde or ketone with the ylide produced by a phosphonium salt. The reactivity of the ylide affects the geometry of the resultant alkene. Georg Wittig invented the Wittig reaction in 1954, for which he received the Nobel Prize in Chemistry 25 years later. It is also known as Wittig olefination.
What is Wittig’s reaction?
Witting reaction is a chemical process that produces alkene from aldehyde or ketone. The Wittig reaction is a crucial step in the synthesis of alkenes. The double bond occurs exactly where the original aldehyde or ketone was. Ylides are neutral molecules with +ve and -ve centers on adjacent atoms linked by a σ bond. Triphenylphosphonium, generally known as the Wittig reagent, is the ylide that was utilized in this reaction.
Wittig olefination stereoselectivity is complicated, but it can be generalized to ylide stability. Stabilized phosphoryl anions produce E-alkenes, whereas non-stabilized anions produce predominantly Z-alkenes. E-alkenes can be obtained from benzylic ylides, which are somewhat stabilized. One possible reason for this selectivity is that if the ylide is stable, the oxaphosphetane or betaine production is reversible, and thus only the faster of the two eliminations can occur to yield the trans isomer.
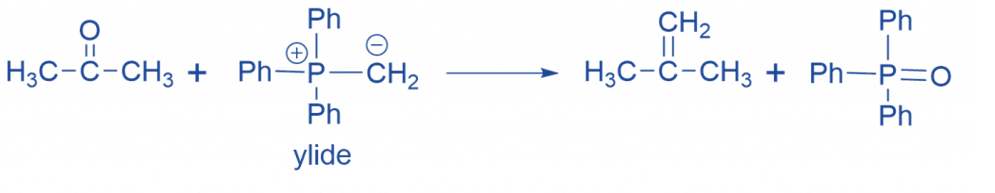
Mechanism of Wittig reaction
Step I: The ylide attacks the aldehyde, producing betaine,
Step II: The betaine cyclizes to generate a stable oxaphosphetane.
Step III: The oxaphosphetane collapses to form the product.
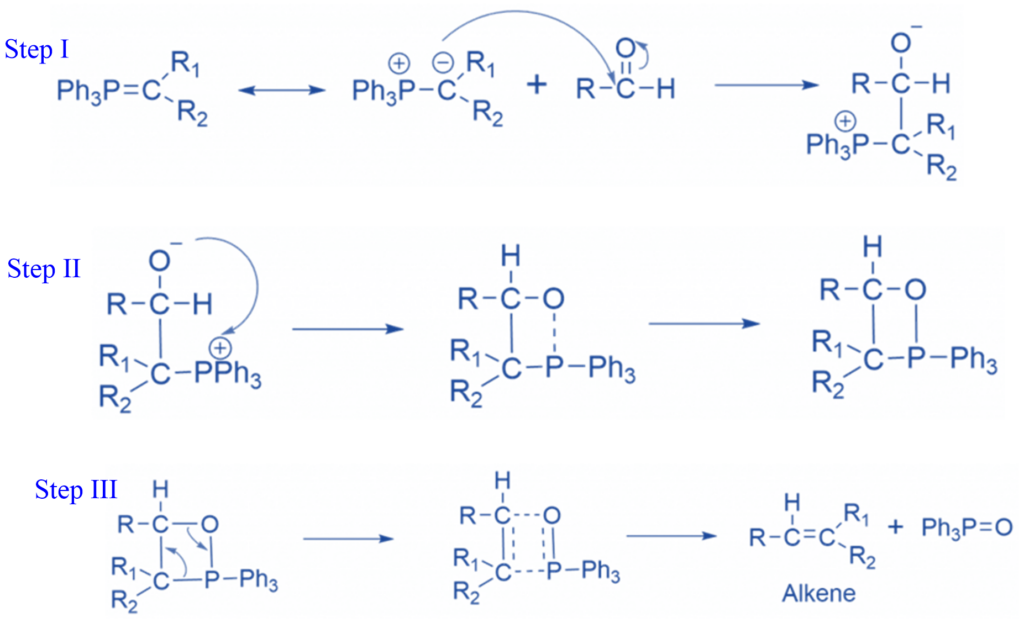
Preparation of Wittig reagent
Triphenyl triphenylphosphine (Ph3P) and an alkyl halide are used to make the Wittig reagent in two phases. First, the Ph3P functions as a nucleophile in the SN2 reaction, replacing the halide to produce a phosphonium salt.
The carbon in the phosphonium salt is linked to a positively charged phosphorus capable of accepting another pair of electrons (P can be greater than the octet). This allows a strong base, such as an organolithium reagent, to remove the hydrogen next to the phosphorous, creating the desired ylid.
Applications of Wittig reaction
- The witting reaction is the most general process for alkene synthesis. Due to the reaction’s selectivity and versatility under mild conditions, it is still a topic worth studying today.
- The Wittig reaction is a vital tool in organic chemistry that is utilized not only in laboratories but also in the industry to synthesize -carotene and vitamin A derivatives.
Limitations of Wittig reaction
- Aldehydes are easily capable of polymerization, oxidation, and decomposition.
- Both the E and Z double-bond isomers can be obtained.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
