The atomic radius of an element is the typical distance from the center of the nucleus to the boundary surrounding the electron cloud. Since the boundary is not a well-defined entity, there are various nonequivalent definitions of atomic radius.
According to J. A Campbell atomic radius in compounds depends on;
1. The multiplicity of the bond i.e., Whether the bond is single, double, or triple.
2. The oxidation number of atoms, indicates the number of electrons involved in bonding.
3. Percentage ionic character of bond.
4. The coordination number of atoms
5. The repulsive forces between atoms, which are not directly bonded with molecules.
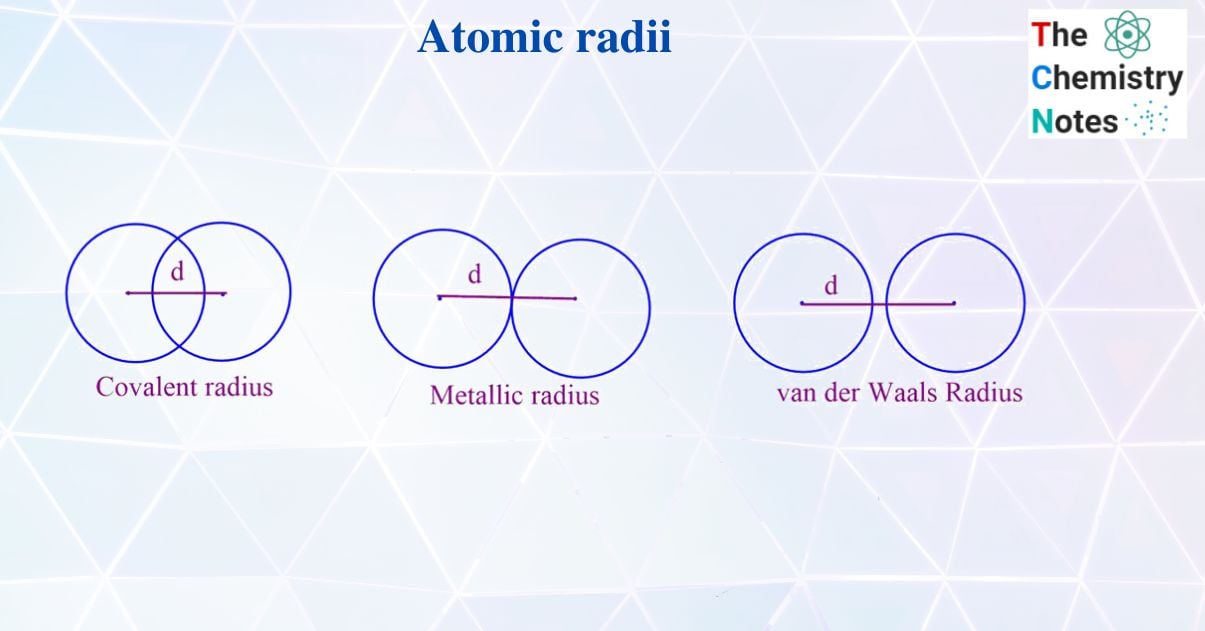
The atomic radius of the element can be best described in terms of covalent radii, van der Waal’s radii, ionic radii, metallic radii, crystal radii, etc.
Covalent radius
Internuclear distance refers to the distance between the nuclei of two similar atoms held together by a single covalent bond. Covalent radii are half the internuclear distance.
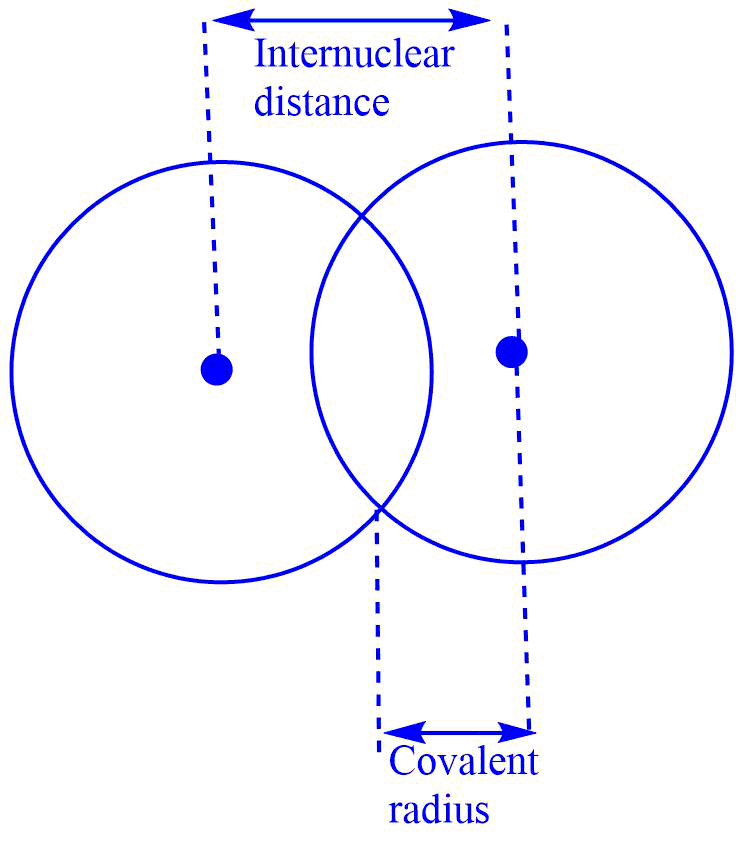
For the homonuclear diatomic molecules (e.g., H2, CI2, F2 etc), the covalent radius can be represented as
Covalent radius = Internuclear distance/ 2
= Bond length/ 2
For the heteroatomic molecules, the inter-nuclear distance is equal to the sum of the covalent radius of two atoms. For example, If the A and B atoms are bonded together by a single covalent bond, the internuclear distance is equal to the covalent radius of A and B.
Internuclear distance (Bond length) = rA + rB
As the internuclear distance decreases from single to double bond to triple bond, the covalent radius also decreases. So, the covalent radii for the single, double and triple bonds are 0.771 Å, O.665 Å, and 0.602 Å respectively. The covalent radii of the element also depend on the geometry of the molecule. For the metal complexes, the radius of metal atoms in different geometry decreases in the order
rtet > roct > rsq
here,
tet = Tetrahedral Fe(NO3)3
oct = Octahedral
sq = Square planar
van der Waal radius
The distance between the nuclei of two adjacent atoms of two same covalent molecules is called intermolecular distance. The half of intermolecular distance is called the van der Waals radius. van der Waal’s radius of the molecule is always larger than that of the covalent radius.
Ionic radius
Ionic radius may be defined as the distance between the center of the nucleus and the point up to which the nucleus influences the electron cloud.
Radius of the cation
A cation is formed by the removal of one or more electrons from the central atom. The cation is always smaller than its parent neutral atom. The radius of the cation decreases if the number of positive charges on the cation increases.
Cr < Cr+ < Cr2+ < Cr3+
As with the removal of electrons, the number of electrons decreases while effective nuclear charge increases which results in an increase in nuclear attraction. Hence the radius decreases.
Radius of anion
Anions are formed by the addition of one or more electrons on the neutral atom. An anion is always larger than the parent neutral atom. The radius of the anion increases if the number of negative charges on the anion increases.
Successive addition of an electron on an atom or ion decreases the effective nuclear charge on the valence shell i.e., the nuclear attraction on valence decreases. In other words, the addition of an electron valence shell cause an increase in inter-electronic repulsion. Hence the electron cloud tends to expand i.e., the ionic radius increases as more electrons are added.
N < N– < N2-< N3-
Radius of isoelectronic species
Atoms or ions having different atomic numbers (i.e., nuclear charge Z ) but the same number of electrons are called isoelectronic species. The radius of isoelectronic species as the atomic number increases.
Example :
| Species | C4- | N3- | O2- | F– |
| Atomic number | 6 | 7 | 8 | 9 |
| Number of electrons | 10 | 10 | 10 | 10 |
Here,
- Number of nuclear charge (Z) increase →
- Number of electrons constant →
- Shielding effect constant →
- Effective nuclear charge (Z eff) increase →
- Nuclear attraction on valence shell increase →
so the radius decrease.
Periodic variation of atomic radius
Variation in s- and p- block element
The atomic radius of the element decreases in the period from left to right. This is due to successive increase in effective nuclear charge on the valence shell, which attracts the valence shell towards the nucleus.
Up to the carbon group elements the atomic radii decreases while after the carbon group the decrease in carbon radii is slow due to increase in the inter- electronic repulsion on the valence shell.
Variation in d-block element
Electrons in d-block elements gradually enter the (n-1) d orbital, i.e., the d- orbital of the penultimate shell, while the valence shell remains unaffected. As electrons on the d- orbital have a low shielding effect, nuclear attraction on the valence shell increases. As a result, the valence shell contracts, and the atomic radii of transition metals decrease from the left to right.
| Elements | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
| Covalent radius (Å) | 1.44 | 1.32 | 1.22 | 1.17 | 1.17 | 1.17 | 1.16 | 1.15 | 1.17 | 1.25 |
However, the atomic radius between Cr and Cu remains nearly constant. Moving from Cr to Cu increases both the nuclear charge and the shielding effect. Increases in nuclear charge tend to reduce atomic radius; however, increases in shielding effect tend to increase the atomic radius. Overall, the shielding effect of d-electrons almost compensates for the increase in nuclear charge. So, the valence shell feels no attraction from the nucleus. As a result, the atomic radius from Cr to Cu remains nearly constant.
Variation in f-block elements
In f-block elements, electrons enter the anti-penultimate shell’s f-orbitals in (n-2) f orbitals, while the penultimate and valence shells remain unaffected. Because f-orbitals have the least shielding effect, the nuclear attraction on the valence shell increases, causing the valence shell to contract and the atomic radius to decrease.
| Elements | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
| Atomic radius (Å) | 1.65 | 1.64 | 1.64 | – | 1.66 | 1.85 | 1.61 | 1.59 | 1.59 | 1.58 | 1.57 | 1.56 | 1.70 | 1.56 |
The atomic radii of f-block elements decrease due to the less shielding effect of orbitals, and also, it is due to the shielding effect exerted by f-orbitals, the variation becomes very slow. As a result, the atomic radii of f-block elements are almost constant and show common physical and chemical properties.
In group
Atomic radii of elements increase on descending a group from to bottom. This is due to the addition of one quantum shell with electrons crossing each period.
However, the elements of group III A (13) show an irregular group trend. Their atomic radii do not increase regularly from top to bottom.
| Elements | B | Al | Ga | In | Tl |
| Covalent radius (Å) | 0.9 | 1.25 | 1.25 | 1.50 | 1.55 |
On moving from B to Al atomic radius increases due to the addition of an extra shell on Al. While descending from Al to Ga there is no substantial increase in atomic radius and their atomic radii are almost the same.
Illustration:
Electronic configuration of Ga = [Ar] 3d10 4S2 4P1
Penultimate shell Valence shell
Gallium (Ga) has ten electrons in d penultimate shell. Since the d orbital electrons have a low shielding effect, the nuclear attraction on the valence shell increases. As a result, the atomic radius decreases and considerably becomes similar to the radius of Al.
On descending from Ga to In, the excessive increase in shielding effect exceeds the increase in nuclear charge, resulting in an increase in the atomic radius.
Going from In to Tl there is no substantial increase in atomic radius.
Electronic configuration of Tl: Ga = [Xe] 4f14 5d10 6S2 6P1
Anti-penultimate shell Penultimate shell Valence shell
Since the d and f orbital have a low shielding effect, the nuclear attraction on the valence shell of Tl increases. As a result, the radius of Tl decreases considerably and becomes similar to the radius of In.
A similar effect is also observed with elements of group IV A (14) and Va (15).
- Lee, J. D. (John David), 1931- New concise inorganic chemistry.
- Cotton, F.A., and Wilkinson, G., Advanced Inorganic Chemistry, Fifth Edition, John Wiley, 1988.
- file:///C:/Users/uSer/Downloads/1589480043-atomic-radii-2.pdf
- https://www.angelo.edu/faculty/kboudrea/periodic/trends_atomicradius.htm
- https://byjus.com/physics/atomic-radii/
- https://www.vedantu.com/chemistry/periodic-trends-in-properties-of-elements
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Atomic_Radii
