Baeyer’s strain theory was developed by Adolf van Baeyer in 1885 to predict the relative satblility of various alicyclic compounds.
This theory is based on van’t Hoff and Le-Bell’s classical hypothesis. This suggests that the carbon atom’s four sigma bonds should be placed in a regular tetrahedron, and as a result, the angle between the two bonds is 109o28′. If the carbon has a bond angle of 109o28′, the molecule is free from any strain and consequently, it is stable.
Baeyer discovered that these cycloalkanes have distinctive bond angles, in addition to having various characteristics and levels of stability. Based on this, he suggested that any deviation from this tetrahedral angle causes strain in the molecule, which lowers the molecule’s stability. The instability increases with an increase in strain. Greater strain results in increased reactivity and heat of combustion. This theory is also known as angle strain theory.
Interesting Science Videos
Postulates of Baeyer’s strain theory
Baeyer’s strain theory is based on the different assumptions. They are as follows:
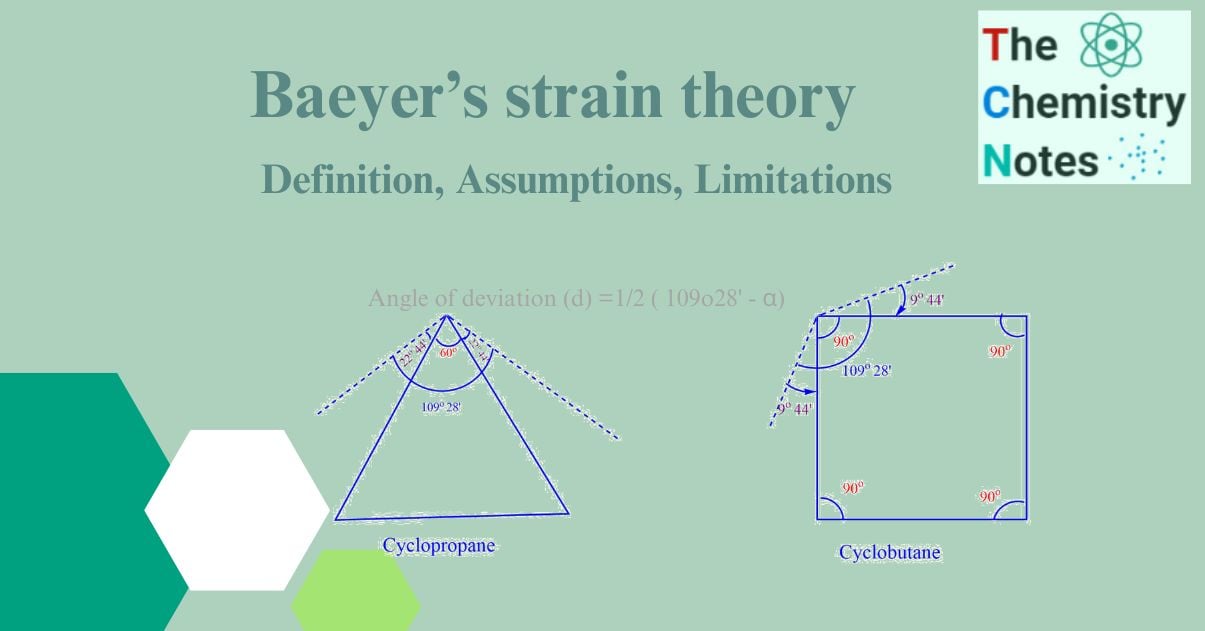
- All cycloalkanes are planar or flat, that is, they all lie in the same plane. As a result, the bond angle between the two adjacent carbon atoms of the ring no longer remains 109o28′. The values of this angle differ between different rings. For instance, the cyclopropane ring is an equilateral triangle with a C-C-C angle of 60o.
- Any variation, positive or negative, from the ideal tetrahedral bond angle of 109o28′ during ring formation causes a strain in the molecule, making it unsuitable. This bond angle deviation is called Baeyer angle strain or simply angle strain.
- The bigger the deviation from the normal angle, the greater the strain and hence the lower the stability. However, the sign of deviation does not make any difference.
- The more stable a ring system is, the easier it is to synthesize.
- The amount of angle strain can be expressed in term of the valence angle deviation ‘d’ which can be mathmatically calculated as,
angle strain (or angle of deviation) d =1/2 ( 109o28′ – α)
where α is the bond angle of various cycloalkanes. The factor ‘1/2’ indicates that the deviation of the angle strain has been assumed to be equally shared between two bonds.
Relative stabilities of cycloalkanes
Cyclopropane
Three carbon atoms make up the corners of an equilateral triangle in cyclopropane. Hence, the C-C-C bond angle of cyclopropane is 60o. This means that a normal tetrahedral angle of 109o28′ between any two bonds is compressed to 60o and each bond is pulled in by angle strain.
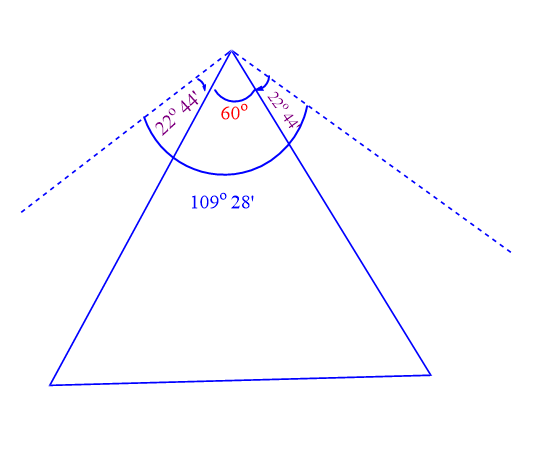
Angle of deviation or angle strain = 1/2 ( 109o28′ – 60o)
= ½ x 49o28′ = 24o28′
The value of 24o28′ then represent the angle strain through which each bond bends from normal tetrahedral direction.
Cyclobutane
Four carbon atoms make up the corners of the square in cyclobutane. Hence, the C-C-C bond angle of cyclopropane is 90 o. This means that a normal tetrahedral angle of 109o28′ between any two bonds is compressed to 90 o and each bond is pulled in by angle strain.
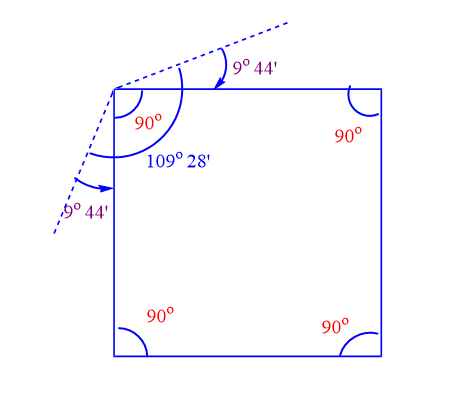
Angle of deviation or angle strain = 1/2 ( 109o28′ – 90o)
= ½ x 19o28′ = 9o44′
The value of 9o44′ then represents the angle strain through which each bond bends from the normal tetrahedral direction.
Cyclopentane
Bayer assumes that the cyclopentane is planar molecule. Thet C- C- C bond angle of cyclopentane is of 108o . It has regular pentagon.
so,
angle strain = 1/2 ( 109o28′ – 108o)
= ½ x 1o28′ = 0o44′.
so angle strain of cyclopentane is negligible.
Cyclohexane
It is assumed that the cyclohexane molecule is planar. So, each C- C- C bond angle becomes 120o like that of regular hexagon.
angle strain = 1/2 ( 109o28′ – 120o)
= ½ x – 10o32′ = -5o16′
This negative bond distortion indicats that cyclohexane has outward bond angle distortion.
So according to this theory, the cyclopentane is the most stable cycloalkane due to lower angle strain.
Angle strain of various ring systems
| No of carbon atom in the ring (n) | Bond angle α = (180o – 360o /n) | Angle strain 1/2 ( 109o28′ – α) |
| 3 (Cyclopropane) | (180o – 360o /3) = 60o | 1/2 ( 109o28′ – 60o) = +24o 44′ |
| 4 (Cyclobutane) | (180o – 360o /4) = 90o | 1/2 ( 109o28′ – 90o) = +9o 44′ |
| 5 (Cyclopentane) | 108o | +0o 44′ |
| 6 (Cyclohexane) | 120o | -5o 16′ |
| 7 (Cycloheptane) | 128o34′ | -9o 33′ |
| 8 (Cyclooctane) | 135o | -12o 46′ |
Positive and negative valence angle deviation ‘d’ values show whether the bond angle is less or greater than the normal tetrahedral bond angle, i.e., whether the strain is inward or outward.
Based on this information, it is obvious that cyclopropane has the greatest angle of strain, as a result, it is the most unstable. Several experimental findings also demonstrate the instability of cyclopropane. It undergoes a ring-opening reaction with H2, Br2, HBr, and so on to release the tension. Furthermore, cyclopropane is difficult to manufacture, which supports the preceding findings.
Cyclobutane has lesser strain than cyclopropane so it is more stable. Only under extreme conditions does it undergo a fission reaction.
Cyclopentane is more stable because of the smaller angle strain. Although cyclohexane has a little higher strain than cyclopentane, it is also found to be highly stable. So, cyclopentane and cyclohexane are stable compounds. They can be easily synthesized and do not undergo ring cleavage.
The cycloalkanes with a ring containing 7 or more carbon, possess a higher angle strain so they are unstable. Baeyer proposed that due to higher strain and their instability, large ring compounds are difficult to synthesize.
Limitation of Baeyer’s Strain Theory
- The effect of angle strain in bigger ring systems could not be well explained by Baeyer’s strain theory.
- According to Baeyer’s strain theory cyclopentane is supposed to be considerably more stable than cyclohexane, but practically it is opposite.
- According to Baeyer’s strain theory, larger ring systems are not possible due to negative strain, although they do exist and are far more stable.
- In order to reduce angle strain, larger ring structures are puckered rather than planar.
Stability of cyclohexane
Baeyer’s strain theory fails to account for the stability of cyclohexane because Baeyer assumed that all atoms of cycloalkanes with six or more carbon atoms lie in the same plane, causing angle strain in the ring and making it unstable. Actually, in cycloalkanes with six or more carbon atoms, all C-atoms are puckered and retain the regular tetrahedral bond angle. Furthermore, cyclohexane’s heat of combustion value per -CH2 (157.4 kcal/mol) indicates that it is more stable than cyclopentane, which has a heat of combustion value per -CH2 of 158.7 kcal/mol.
Heat of combustion and relative stabilities of cycloalkanes
On the basis of the heat of combustion per -CH2, it is feasible to study the relative stability of cycloalkanes. Heat of combustion is the amount of energy released when one mole of a substance is burned to produce CO2 and water. Study of the heat of combustion data for large compounds revealed that the heat of the combustion per -CH2 of different members of cycloalkanes are very close with combustion value of open chain compounds i.e. -157.4 kcal/mol. In general, the stability of a compound with a high heat of combustion will be low, and vice versa.
Heat of combustion of cycloalkanes
| Ring size | Heat of combustion per -CH2 group in kcal/mol |
| 3 | 166.6 |
| 4 | 164 |
| 5 | 158.7 |
| 6 | 157.4 |
| 7 | 158.3 |
| 8 | 158.6 |
| 9 | 158.8 |
| 10 | 158.6 |
| 11 | 158.4 |
| 12 | 157.6 |
| 13 | 157.8 |
| 14 | 157.4 |
| open chain | 157.4 |
From the heat of combustion data, we can easily drawn following conclusions
- In comparison to open chain molecules, cyclopropane and cyclobutane evolve more energy per -CH2 group. As a result, cyclopropane and cyclobutane are less stable than open chain compounds, and it is possible that their tendency to undergo ring opening reaction is connected to their instaclility. This supports Baeyer’s strain theory.
- According to Baeyer’s strain theory, cyclopentane is the most stable, but cyclohexane should be unstable due to outward bond distortion (-5o16′). Nonetheless, cyclohexane’s heat of combustion value per -CH2 of 157.4 kcal/mol suggests that cyclohexane is more stable than cyclopentane, whose heat of combustion value per -CH2 is 158.7 kcal/mol. Thus, Beayer’s strain theory is unable to explain cyclohexane’s stability.
- Baeyer suggested that rings that are bigger than cyclopentane and cyclohexane may be unstable and hence have a high heat of combustion. Additionally, due to an increase in angle strain, the heat of combustion should rise gradually with ring size. But so far, the heat of combustion data indicated that the stability of all cycloalknes with more than four carbon atoms is comparable to that of open chain alkanes. This is contrary to the outcome that would be expected for a larger ring based on Baeyer’s strain theory. Lately, it has been possible to create alicyclic rings with 17 or more carbon atoms. Again, this contradicts Baeyer’s strain theory.
For more information follow this link:
References
- Michael B. Smith, March’s Advanced Organic Chemistry, (7th Edition), John Wiley and Sons, Inc., 2013.
- http://www.vpscience.org/materials/US02CCHE01Baeyer%20strain%20theory.pdf
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- https://www.vedantu.com/chemistry/strain-theory
- J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992
