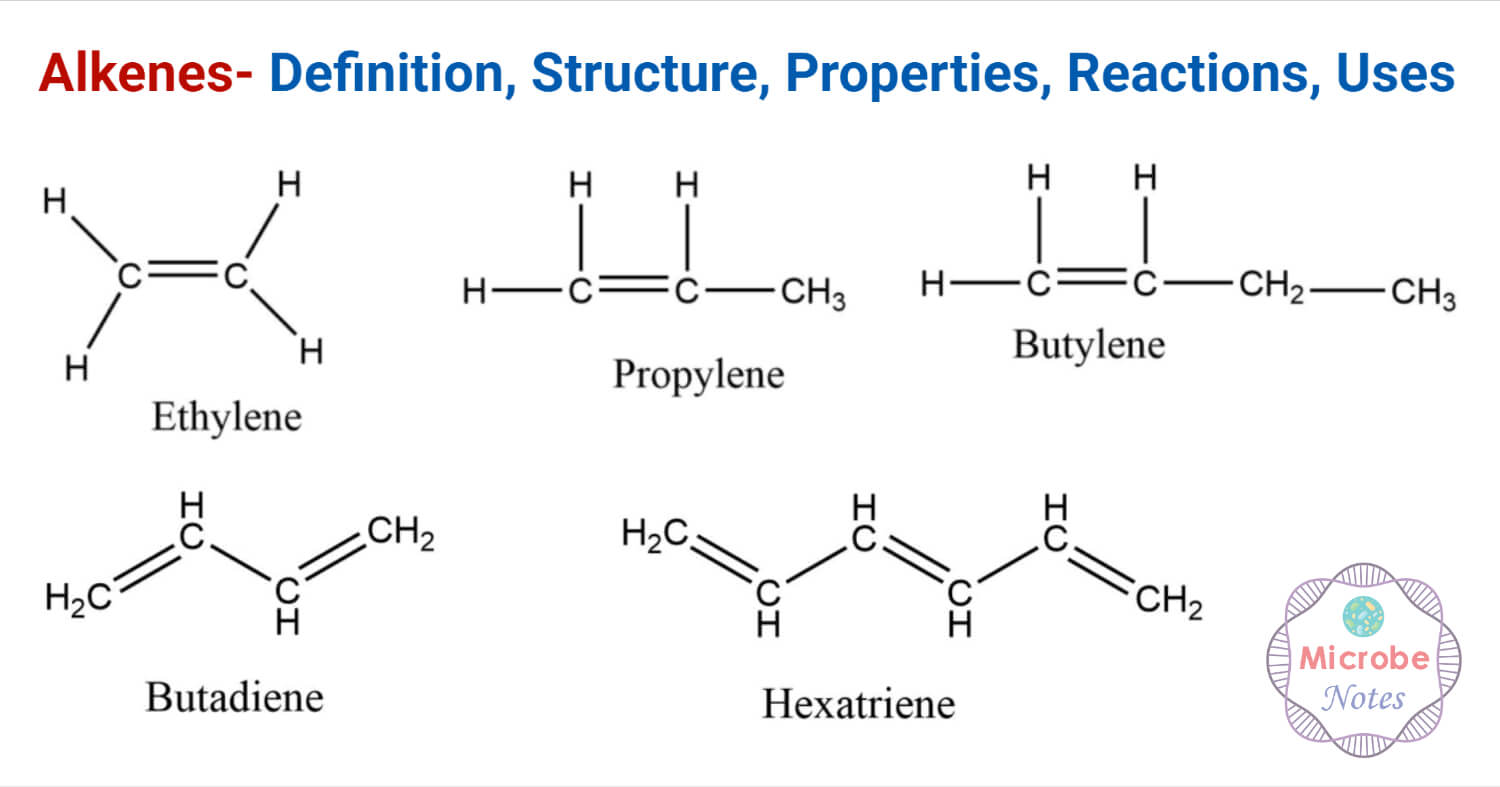
Alkenes are unsaturated hydrocarbons containing carbon-carbon double bonds.
The carbon-carbon double bond is the functional group for alkenes, known as ethylenic linkage.
The general formula of an alkene is CnH2n, where n is the integer number. Alkenes consist of two atoms of hydrogen less than the alkanes.
The chemical properties of alkenes depend on the double bonds present in the alkenes. Generally, alkenes show addition reactions with different reagents.
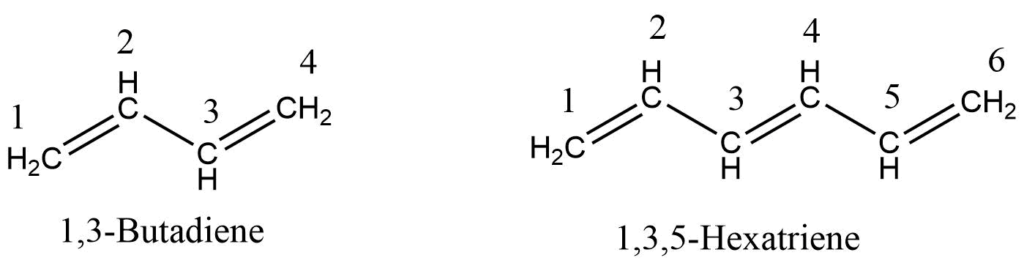
Compounds containing two double bonds are simply called diene or alkadienes while compounds containing three, four, or many double bonds are called triene, tetraene, and polyene respectively. The compounds having alternate single and double bonds are called conjugated compounds.
Alkenes Structure
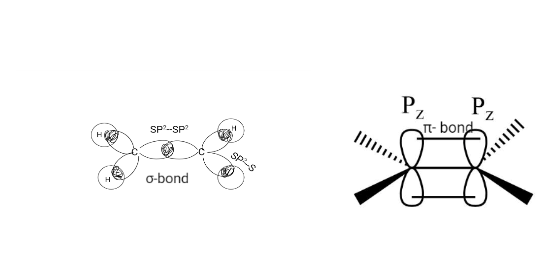
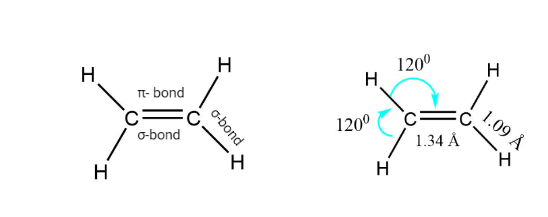
Alkene consists of sp2 hybridized double-bonded carbon atoms. The σ-bond is formed by the overlapping of the SP2 hybrid orbitals, whereas the π- bond is generated by the lateral overlapping of the unhybridized Pz orbital of one carbon atom to another. The π bond is weaker than the σ- bond.
Alkenes Nomenclature
There are two ways of naming alkanes.
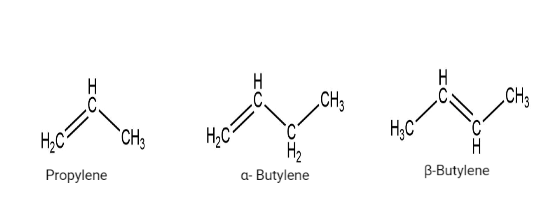
Common name system
In this system, alkanes are named by changing the ending -ane of corresponding alkanes by -ylene. Greek letters are used to distinguish isomeric alkenes.
IUPAC system
- IUPAC names of alkanes are derived from corresponding alkanes by changing the ending -ane to -ene.
- The name of hydrocarbon is based on the parent alkene having the longest carbon chain.
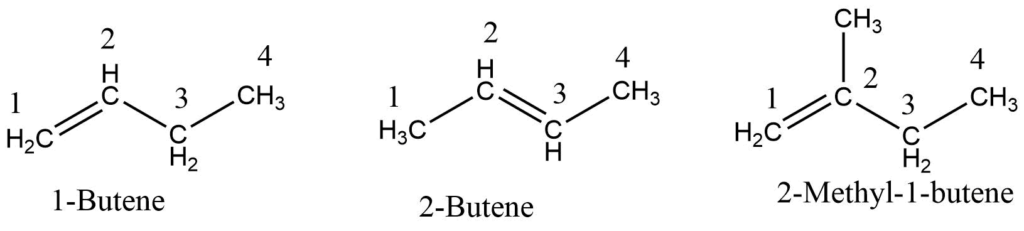
- The chain is numbered from the end near the double bond, and its position is indicated by the number of a carbon atom at which the double bond originates.
- The position of other substituents is specified with the appropriate number, and its name is prefixed to the parent alkene.
- If a molecule has two or three double bonds, the corresponding alkane’s ending -ane is changed with -adiene, -atriene to form the name of hydrocarbon.
Alkenyl group
Alkenyl group is obtained by removing one hydrogen atom from an alkene. IUPAC name of the alkenyl group is given by replacing -ne of parent alkene with -yl. The carbon with free valence is always numbered first.

Isomerism
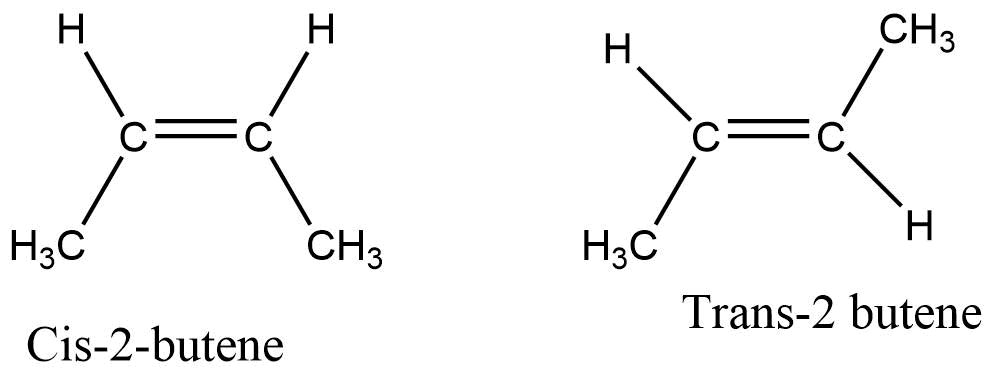
Alkene containing four or more carbon has positional and chain isomers. As the π- bond prevents the free rotation about the double bond this prevention of rotation about the carbon-carbon double bond gives rise to the phenomenon of geometrical isomerism.
Substituted alkene gives two types of stereoisomers depending upon where the two alkyl groups are on the same side or opposite side of the double bond. Cis isomer is obtained when two groups are on the same side of the double bond, while the alkyl group present on the opposite side of the double bond gives the trans isomer.

Here, 1-Butene and 2-butene are positional isomers where the position of the double bond is different. 2-Methylpropene is the chain isomers of 1-Butene (or 2- Butene) which consist of different chain length of the carbon.
General methods of preparation of alkenes
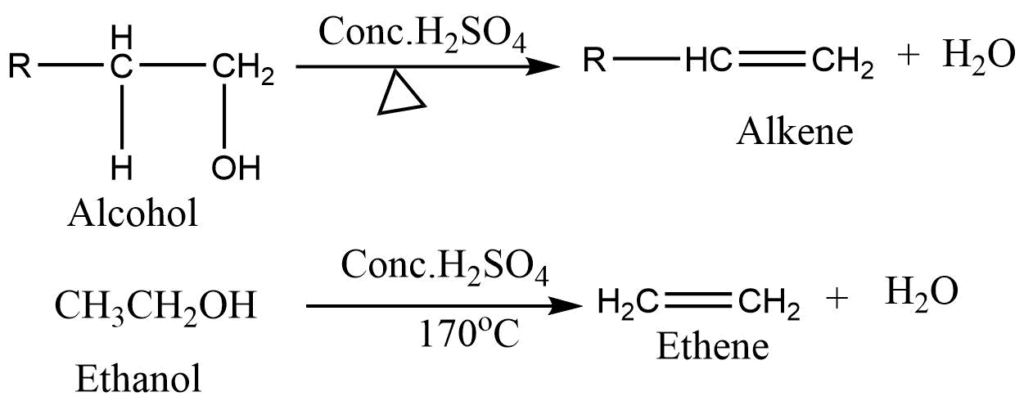
1. By dehydration of alcohol: Removal of the water molecule from compounds is known as dehydration. Alcohol when heated with the acid catalyst like Conc.H2SO4, H3PO4, or Al2O3, alkene is obtained. Unsymmetrical alcohols elimination proceeds in two ways and the mixture of alkene is formed. The formation of a major product is according to the Saytzeff rule.
(Saytzeff rule: In the elimination of unsaturated alcohols the hydrogen is preferentially eliminated from carbon atoms with a fewer number of hydrogen atoms.)
2. Dehydrohalogenation of alkyl halide: Alkyl halide on heating with alcoholic potassium hydroxide alkene is formed and a hydrogen halide is obtained as a side product. This reaction also follows Saytzeff rule. The order of dehydrogenation of alkyl halides is: Iodide> Bromide>Chloride
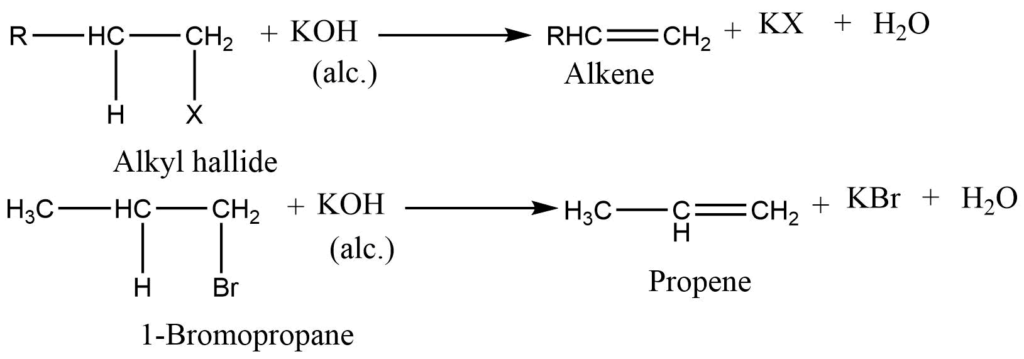
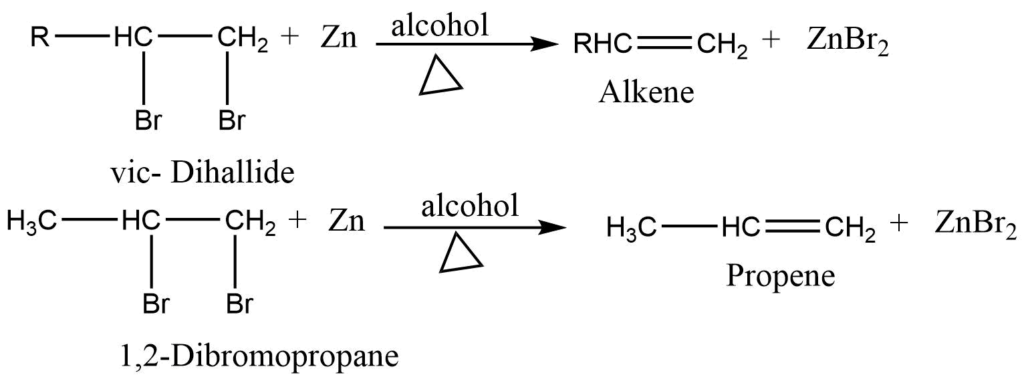
3. Dehalogenation of vicinal dihalides: When vicinal dihalide is heated with Zn dust in alcohol, an alkene is obtained.
(Vicinal dihalide: compounds containing halogen group on adjacent carbon atoms.)
4. By controlled hydrogenation of alkynes: Reaction of alkynes with Na or Li in liquid NH3 or with hydrogen in presence of Lindlar’s catalyst gives alkene.
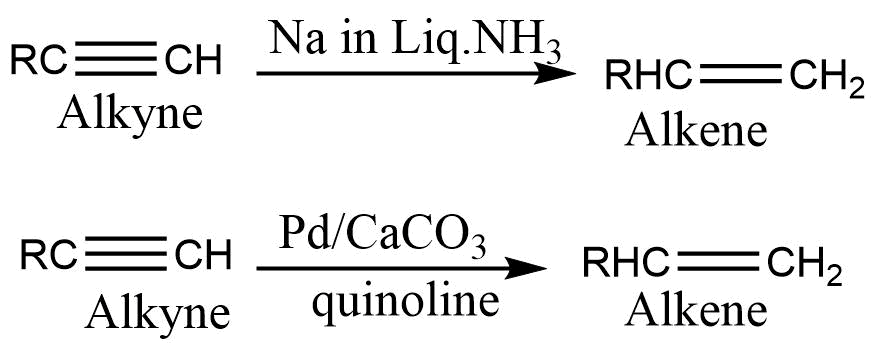
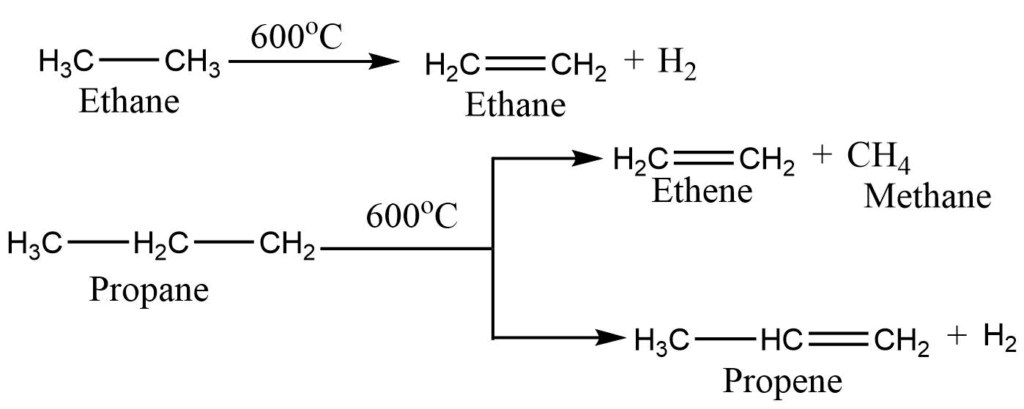
5. By cracking of alkanes: On heating of alkanes at 500-8000C in absence of air gives lower molecular weight alkene, alkanes, and hydrogen. The reaction can be performed at a lower temperature in presence of the catalyst, i.e., known as catalytic cracking.
6. By Kolbe’s electrolysis: On electrolysis of potassium succinate in between platinum electrode ethene is formed at the anode. This is called Kolbe’s electrolysis.
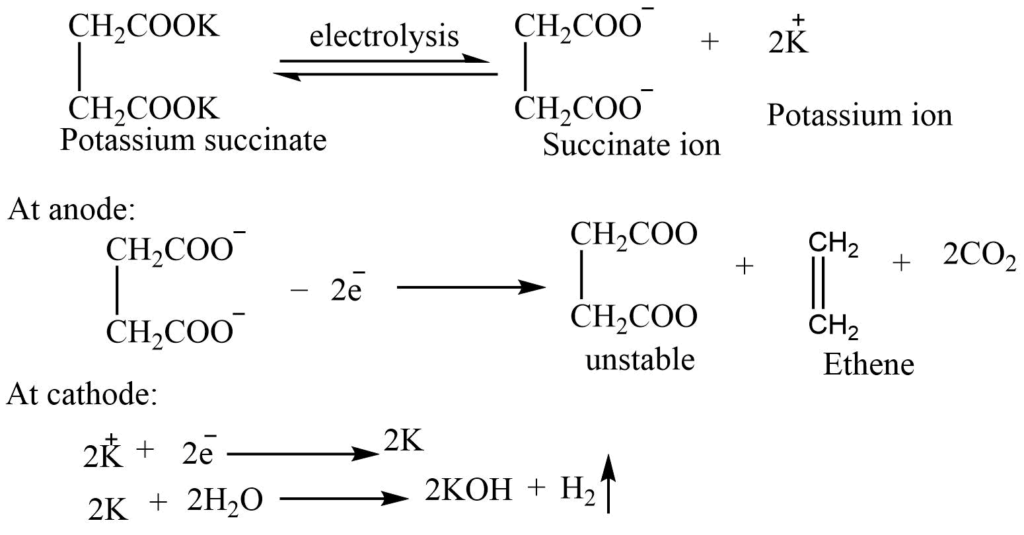
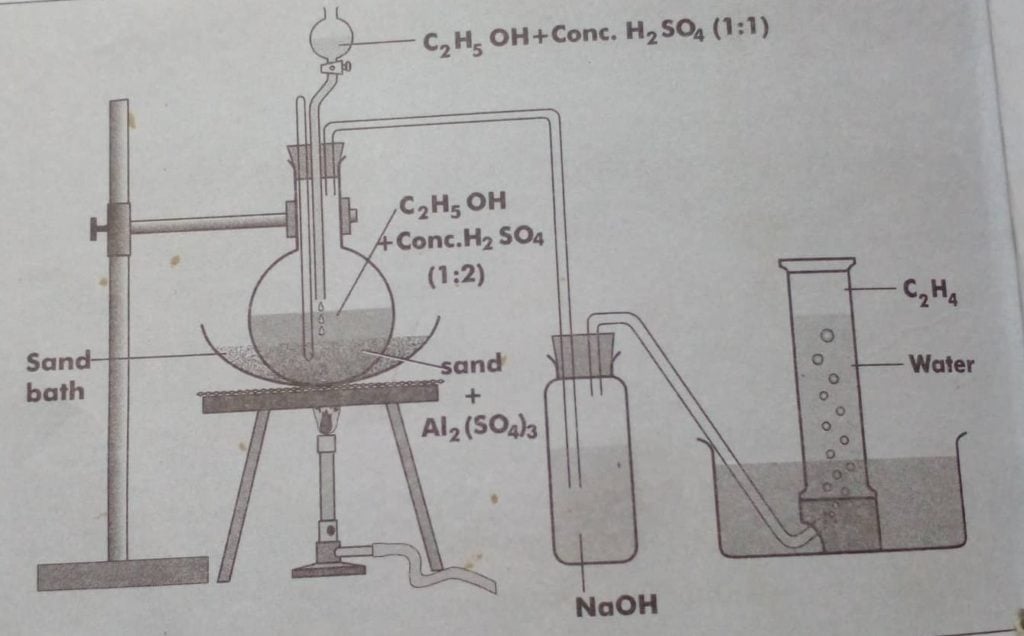
Laboratory preparation of ethene
Ethene can be prepared in the laboratory by dehydration of ethanol. Ethanol on heating with excess of conc. H2SO4 at 170oC, produces alkene.
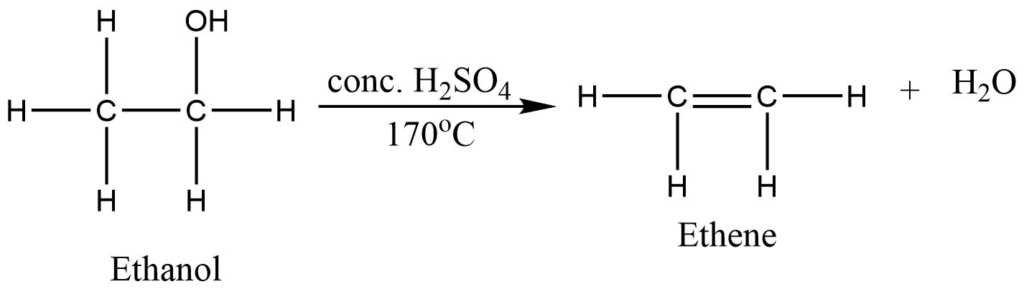
Step 1: Reaction of ethyl alcohol with sulphuric acid produces ethyl hydrogen sulphate
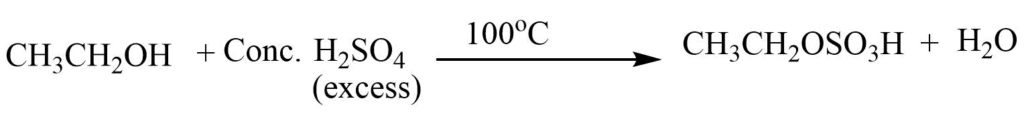
Step 2: Ethyl hydrogen sulphate decomposes at 170oC to give ethene
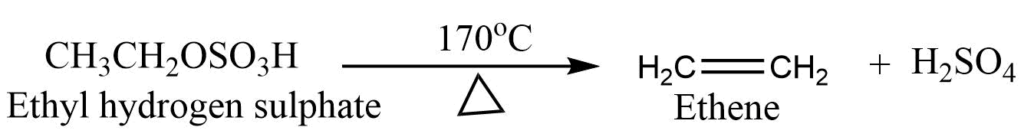
When excess of alcohol is used ethoxy ethane is produced.
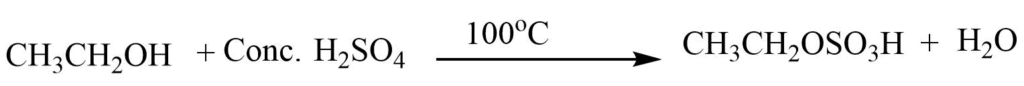
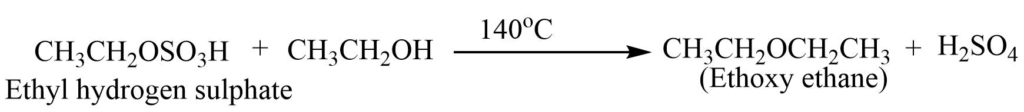
Physical properties of Alkenes
- Lower members of alkenes are gases while higher members are liquids. The alkenes containing more than 18 carbon in the molecule are solids.
- They are colorless and odorless except for ethene. Ethene has a faintly sweet odor.
- Alkenes are insoluble in water and are readily soluble in organic solvents.
- Generally, their melting point, boiling point, and specific gravity increase with an increase in molecular weight in homologous series.
- Alkenes are less volatile than corresponding alkanes.
- IR spectrum of alkene shows C-H stretching absorption at 3000-3100 cm-1, C=C stretching absorption at 1620- 1680 cm-1, and C-H bending vibration at 600-1000 cm-1.
Chemical properties of Alkenes
Alkenes are more reactive than alkanes. The higher reactivity of the alkene is due to the presence of a weak pi bond in the carbon-carbon double bond. There is no effective overlapping of atomic orbitals while forming the pi bond. so, the π- bond is weaker than the sigma bond. some reactions of alkenes are:
Addition reactions
1. Addition of hydrogen: In presence of a catalyst like Ni, Pt, or Pd alkene adds the hydrogen to give alkane. This is also called catalytic hydrogenation. This process depends on the affinity of hydrogen toward the metal catalyst. Usually, cis addition is observed in catalytic hydrogenation due to the addition of hydrogen from the same side of the carbon-carbon double bond.
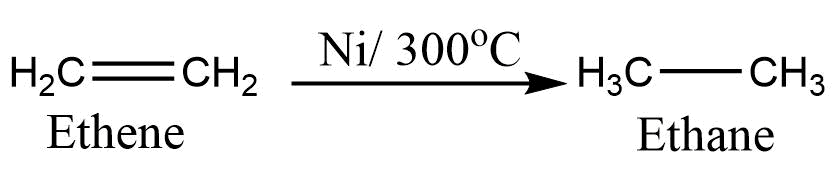
2. Addition of halogen: Alkene reacts with halogen in presence of an inert solvent to form dihalogen derivatives.
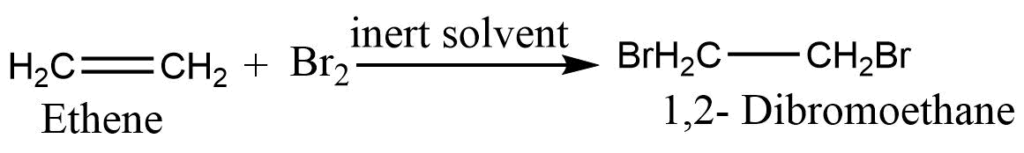
In this reaction, the reddish-brown color of bromine gets discharged to give colorless 1,2-Dibromoethane showing a positive unsaturation test.
3. Addition of hydrogen halide (halo acid): Alkene reacts with hydrogen halide to give haloalkane. This reaction is also nown as the hydrohalogenation reaction.
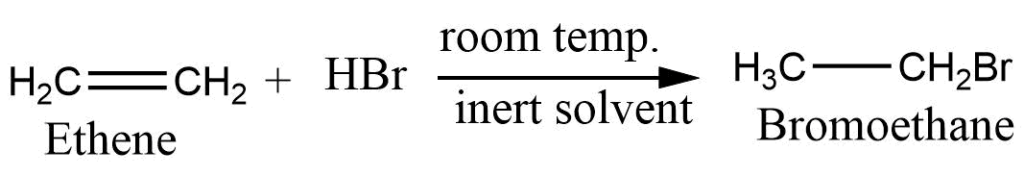
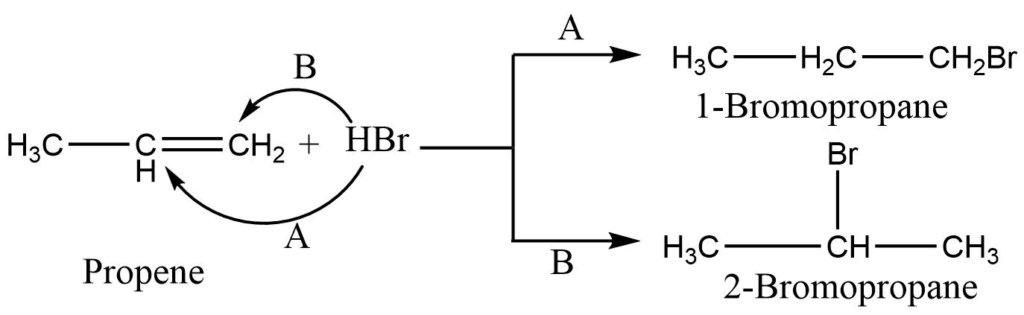
The addition of haloacid to the unsymmetrical alkene takes place according to Markovnikov’s rule. (Markovnikov’s rule: When an unsymmetrical reagent adds to the unsymmetrical alkene, the positive part of the reagent becomes attached to double-bonded carbon which have the greatest number of hydrogen atoms.)
4. Addition of sulphuric acid: Alkene on reaction with sulphuric acid gives alkyl hydrogen sulphate, which on hydrolysis gives alcohol.
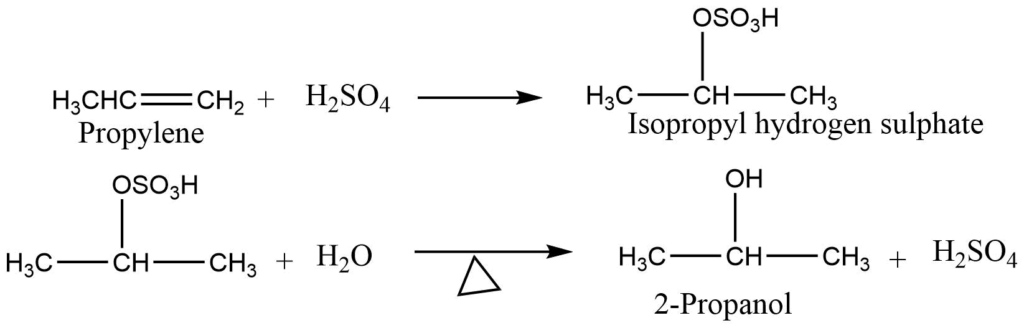
5. Addition of water: Ethene takes up a molecule of water in presence of phosphoric acid at high temperature and pressure to form ethanol.
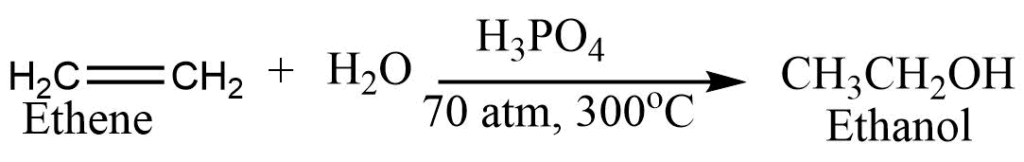
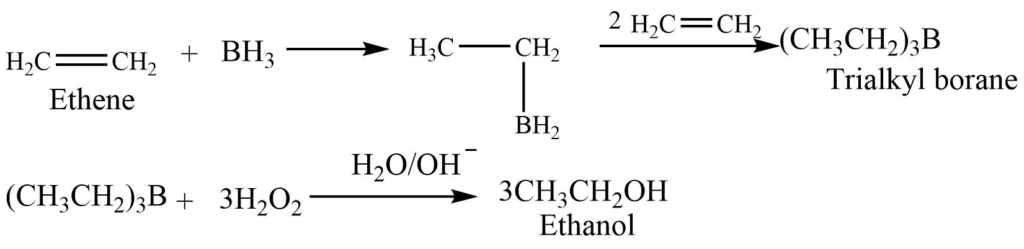
Hydroboration-Oxidation reaction
Diborane reacts with an alkene to give trialkyl borane. Trialkyl borane on oxidation with hydrogen peroxide gives alcohol.
Oxidation reactions
1. Combustion reaction: Alkene burns in air to give CO2 and water.
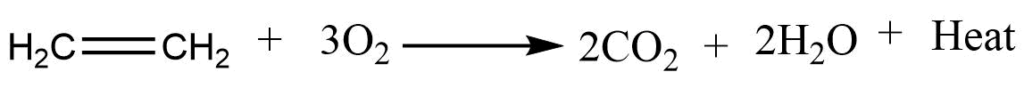
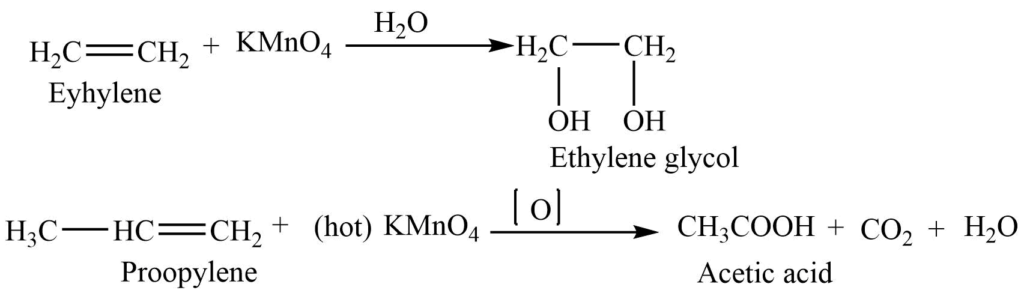
2. Oxidation with KMnO4: Alkene reacts with alkaline potassium permanganate (KMnO4)to give glycol. Where the pink color of potassium permanganate is discharged. So this reaction is used to test for unsaturation test (Baeyer’s test)
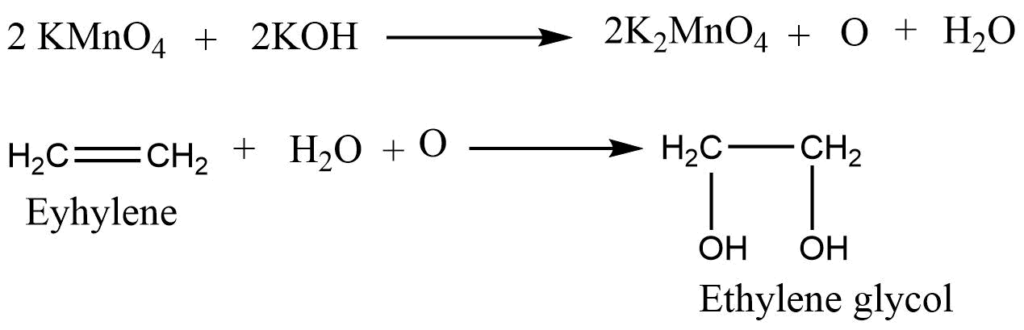
Cold dilute potassium permanganate solution reacts with an alkene to form glycol, whereas hot concentrated potassium permanganate reacts with an alkene to form ketones and/or acids.
3. Catalytic oxidation: At 250-400oC, alkene reacts with oxygen in presence of a silver catalyst to form an epoxide.
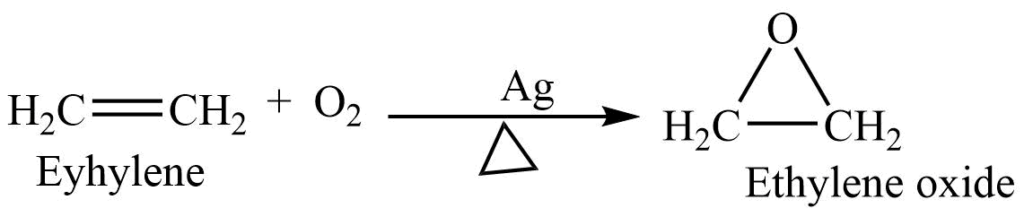
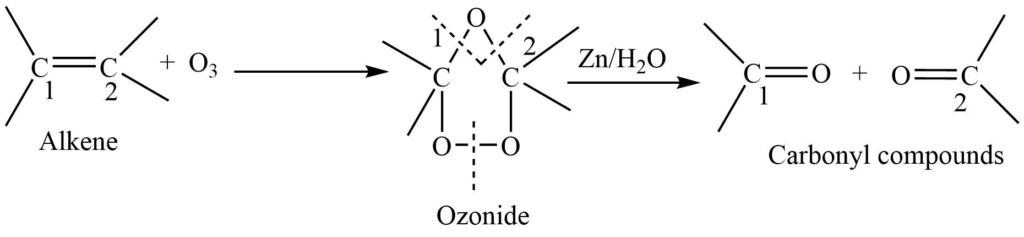
4. Oxidation with Ozone: The reaction of an alkene with ozone in an inert solvent gives ozonide. Ozonide, on warming with zinc and water cleaves to produce aldehyde, ketones, or an aldehyde and a ketone depending on the nature of the alkene.
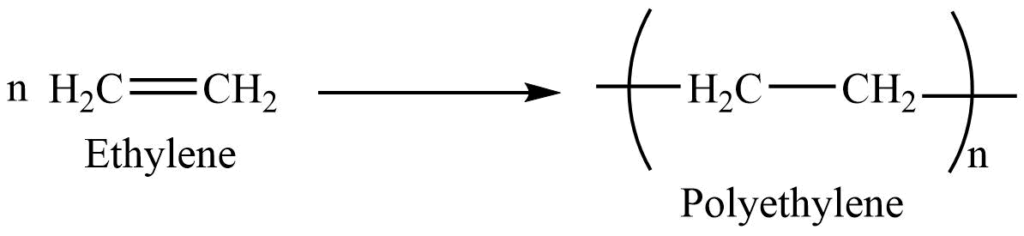
5. Polymerization reaction: Polymerization is the process by which simple molecules with low molecular weight are joined together to produce large molecules of high molecular weight. In the presence of a catalyst such as HF, H2SO4, or organic epoxide, alkenes polymerize to form long-chain polymers. The reaction normally takes place at high temperatures and pressures.
6. Allylic substitution: When an alkene reacts with Cl2 or Br2 at a high temperature, one of its allylic hydrogens is replaced by a halogen atom, giving a substituted product.
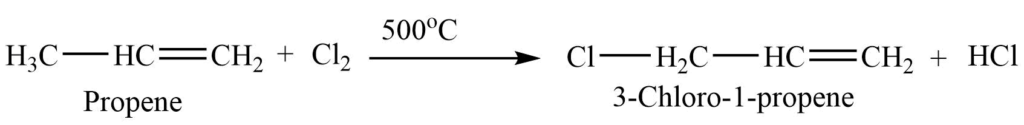
Alkenes Uses
- Alkenes are used as the building block to prepare complex organic compounds.
- Alkenes are used for the manufacture of polyethylene, which is used for making polythene pipes, buckets, and toys.
- Ethylene oxide obtained from ethylene, is used as cellosolves.
- Ethylene glycol obtained from ethene is used as an anti-freeze agent in automobile radiators.
- Ethene is used as the artificial ripening of fruits and as an anesthetic.
- Ethene acts as starting material for the preparation of ethanol, which acts as a solvent and constituent of cleaning preparations.
- Oxy-ethylene derived from ethylene is utilized in the production of mustard gas, alcohol, and its flame for metal cutting and welding.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- https://byjus.com/chemistry/alkene/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/07%3A_Alkenes_Structure_and_Reactivity/7.02%3A_Industrial_Preparation_and_Use_of_Alkene.
- https://www.brainkart.com/article/Chemical-properties-of-alkenes_36497/
- https://www.embibe.com/exams/alkenes/
- https://www.britannica.com/science/hydrocarbon/Nomenclature-of-alkenes-and-alkynes
- https://www.angelo.edu/faculty/kboudrea/index_2353/Chapter_02_6SPP.pdf
