An electrophilic substitution reaction is a chemical process in which an electrophile replaces the functional group connected to a molecule. The displaced functional group is usually a hydrogen atom.
The primary distinction between nucleophilic and electrophilic substitution reactions is that the former requires the displacement of a leaving group by a nucleophile, whereas the latter involves the displacement of a functional group by an electrophile.
Mechanism of electrophilic substitution reaction
Electrophilic substitution reaction mainly involves three steps:
- Generation of electrophile
- Formation of intermediate
- Abstraction of hydrogen from intermediate
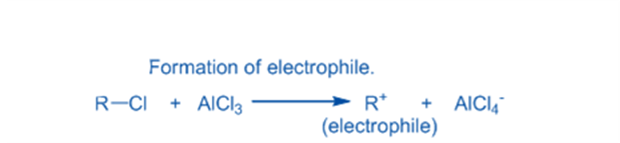
Generation of electrophile
Anhydrous aluminum chloride is a very helpful Lewis acid in the production of electrophiles for aromatic ring chlorination, alkylation, and acylation. Electrophiles are formed in the presence of Lewis acid. The attacking reagent’s electron pair is accepted by the Lewis acid. Cl+, R+, and RC+O electrophiles are generated from the conjunction of anhydrous aluminum chloride with the attacking reagent.
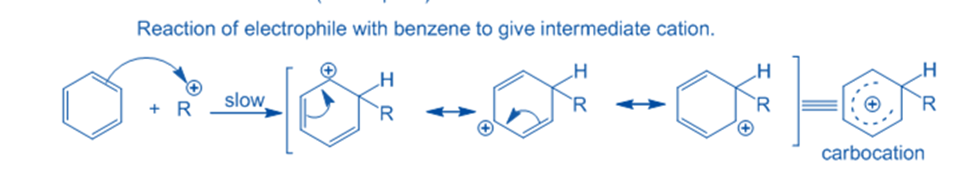
Formation of intermediate
When an electrophile attacks an aromatic ring, an arenium ion or sigma complex is formed. Another carbon in this arenium ion has gone through sp3 hybridization. This arenium ion achieves stability in a resonance configuration. Because electron delocalization occurs at the sp3 hybridized carbon,’ the aromatic property of the sigma complex or intermediate ion’ is lost.
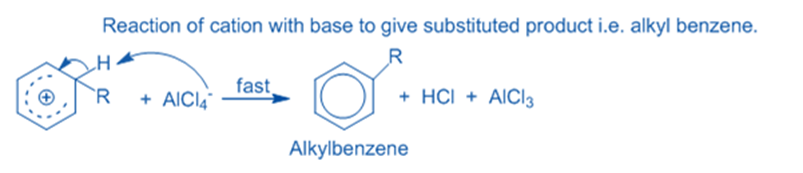
Abstraction of hydrogen from intermediate
The sigma complex releases a proton from the sp3 hybridized carbon when it is attacked by AlCl4 to restore the aromatic characteristic. As a result, the electrophile replaces the hydrogen ion within the benzene ring. Because the concept of electrophilic substitution has been applied in the majority of organic name reactions, it is an extremely important reaction in organic chemistry.
Types of electrophilic substitution reaction
There are two types of electrophilic substitution reaction processes. They are:
- Electrophilic aliphatic substitution reaction
- Electrophilic Aromatic substitution reaction
Electrophilic aliphatic substitution reaction
‘Electrophilic aliphatic substitution reactions’ occur when an electrophile displaces a functional group in an aliphatic molecule. In these reactions, the electrophile attacks the aliphatic molecule, resulting in a 180° inversion. The electrophilic substitution in aliphatic compounds is identical to the nucleophilic substitution in aliphatic compounds, except that an electrophile replaces a functional group rather than a nucleophile. The two basic electrophilic substitutions are SE2 (substitution electrophilic bimolecular) and SEi (substitution electrophilic internal) processes.
Substitution electrophilic bimolecular(SE2)
Bimolecular electrophilic substitution (SE2) reactions are essentially chemical changes in which a stronger electrophile displaces a weaker electrophile in an aliphatic substrate.
Except for the form of attack, this technique is very similar to the SN2 route. Due to its inter-cloud repulsion with the leaving group, a stronger nucleophile replaces a weaker one via the backside attack in the SN2 mechanism; however, in the SE2 route, the attacking electrophile may come from the front as well as the backside because it is only using its vacant orbital towards substrates, causing little to no repulsion. So, depending on the front and rear attacks, the SE2 mechanism can be separated into SE2-front and SE2-back.
Substitution electrophilic internal (SEi)
Internal electrophilic substitution reactions are chemical changes that occur when a stronger electrophile displaces a weaker one in an aliphatic substrate by facilitating its departure.
Except for the form of attack, this technique is extremely similar to the SN2 route. Due to its inter-cloud repulsion with the leaving group, a stronger nucleophile replaces a weaker one via the backside attack in the SN2 mechanism; whereas, in the SEI pathway, the attacking electrophile comes from the front and facilitates the leaving group’s departure by establishing a bond with it.
Examples of electrophilic aliphatic substitution reactions are:
- Nitrosation
- Ketone halogenation
- Carbene insertion into a carbon-hydrogen bond
- Tautomerism of keto-enol
- Diazonium fusion (aliphatic)
Electrophilic aromatic substitution reaction
An aromatic compound undergoes an electrophilic substitution reaction when the aromatic ring is substituted or displaced by an electrophile. In such reactions, the aromaticity of molecules is retained. Examples of electrophilic aromatic substitution reactions include sulfonation, Friedel-Crafts reactions, and nitration in aromatic compounds.
Importance of electrophilic substitution reactions
- One of the most important procedures in synthetic organic chemistry is electrophilic aromatic substitution.
- These reactions produce a significant intermediate that can be used as a precursor in the manufacture of industrial, agrochemical, and medicinal products.
- In mild conditions, zeolites can benefit from halogenation, alkylation, para-regioselective nitration, acylation, and methanesulfonyl reaction pathways. They are also typically simple to filter out of the reaction mixture and then revitalize by heating, enabling recurrent use with nearly the same productivity and selectivity as samples.
References
- https://www.dalalinstitute.com/wp-content/uploads/Books/A-Textbook-of-Organic-Chemistry-Volume-1/ATOOCV1-7-0-Aliphatic-Electrophilic-Substitution.pdf
- https://byjus.com/chemistry/electrophilic-substitution-reaction-mechanism/
- https://www.chemistrylearner.com/electrophilic-substitution.html
- https://collegedunia.com/exams/electrophilic-substitution-definition-reaction-and-mechanism-chemistry-articleid-1947
- https://unacademy.com/content/jee/study-material/chemistry/electrophilic-substitution-reaction/
