Halogenation of alkane involves the replacement of one or more hydrogen atoms in organic compounds by halogen atoms (like F, Cl, Br, I).
It is the simple substitution reaction in which a C – H bond is broken and a new C – X bond is formed. It can be carried out under controlled conditions. This constitutes an important industrial process for the preparation of haloalkane. Halogens like chlorine and bromine don’t react with alkane in dark conditions but do in presence of U.V light or at high temperatures (250 – 400O C).
Reaction involved:
CnH2n+2 + X2 → CnH2n-1 + HX
CH4 + X2 → CH3 – X+ HX (at 250 – 400O C)
Interesting Science Videos
Mechanism of the halogenation reaction
The halogenation of alkanes follows a free radical mechanism. It involves three steps i.e. Chain initiation, Chain propagation, and chain termination.
Chain initiation:
In presence of heat or sunlight halogen absorbs the energy and undergoes homolytic fission producing a halogen-free radical.
X – X → 2X.
Chain propagation:
A halogen, free radical abstracts hydrogen from the alkane R-H to form an alkyl free radical. Which in turn abstracts a halogen atom from the halogen molecule to give alkyl halide.
X. + R – H → R. + H – X (rate determining step)
R. + X – X → R – X + X.
These steps are repeated until finally a chain is terminated.
Chain termination step:
The chain reactions come to end if the free radical combines amongst themselves to form neutral molecules.
X. + X. → X – X
R. + X. → R – X
R. + R. → R – R
The formation of alkyl halides depends on the formation of an alkyl radical, which in turn depends upon the alkane and abstracted hydrogen atom. The rate of halogenation reaction depends upon how fast the alkyl radical is formed. Therefore, the formation of an alkyl radial is a slow step that controls the rate of the overall reaction.
Evidence of free radical mechanism
Unlike nucleophilic substitution, which uses two different mechanisms depending on the starting material and reagent, all alkane halogenation reactions use the same mechanism regardless of the halogen and alkane used. There are certain shreds of evidence that support the free radical mechanism:
1. The reaction does not take place in the dark at room temperature. Light heat or additional peroxide is required for the initiation of the reaction. Light and heat provide the energy required to break the X – X bond into radicals, and breaking the peroxides’ weak O – O bond also initiates radical reactions.
2. The reaction has a high yield i.e., numerous alkyl halide molecules are formed for each photon of light absorbed. This fact can be explained on the basis of the chain propagation step.
3. Oxygen acts as an inhibitor. Diradical O2 reacts with alkyl free radicals to form peroxyalkyl radical R – O – O. . This is much less reactive than the alkyl free radical to undergo the chain reaction. As a result, the halogenation of alkane in the presence of oxygen is slowed down or stopped
4. Addition of the substances which are a source of free radicals should initiate the reaction even in dark at room temperature. For example, the chlorination of methane can be carried out in dark at room temperature in presence of a small amount of benzoyl chloride.
Ease of abstraction of the halogen atom
In the halogenation of alkane, the rate-determining step is an abstraction of a hydrogen atom by a halogen atom. The product formed is the hydrogen halide and alkyl free radical.
R – H + X. → R. + H – X (rate determining step)
The relative rate with which the different classes of the hydrogen atom are abstracted is 30 > 20 > 10 > CH3 – H. This order applies to the various hydrogens within a single alkane and governs the orientation of the reaction. Similarly, Similarly, this order applies the various alkanes and thus governs the relative reactivities.
The rate of abstraction among primary, secondary, and tertiary hydrogens is due to differences in Eact. The energy of activation of various alkanes is listed in the table below:
| R | X = Cl | X = Br |
| CH3 | 4 | 18 |
| 10 | 1 | 13 |
| 20 | 0.5 | 10 |
| 30 | 0.1 | 7.5 |
The table shows Eac t. for chlorination and bromination increases when the abstraction of the hydrogen atom of alkane goes from 30, 20, 10, and CH3-H. In chlorination, the difference in Eac t like the differences in rate (5: 3.8: 1) is small while in bromination both differences i.e., rate (1600: 82: 1) and Eact, are large. The rise of temperature speeds up the abstraction of primary hydrogens (with the largest Eac t) and the abstraction of tertiary hydrogens (with the smallest Eac t) is the least and three classes of hydrogens display nearly the same reactivity.
Ease of formation of free radicals
The abstraction of a hydrogen atom from an alkane produces alkyl free radicals. Abstraction of primary hydrogen from alkane yields a primary radical, abstraction of secondary hydrogen from alkane yields a secondary radical, and so on.
CH3 – H + X. → CH3. (Primary radical) + HX
(CH3)2 CH- H + X. → C(CH3)2 CH. (Secondary radical) + HX
And so on.
As the ease of abstraction of hydrogen follows the 30 > 20 > 10 > CH3 – H. Therefore, the ease of formation of order free radical must follow the same or30 > 20 > 10 > CH3 – H
Reactivity of halogen towards halogenation reaction
The reactivity of halogens decreases in the following order:
F2 > Cl2 > Br2 > I2.
Fluorine is explosively reactive to be employed as a halogenated reagent. It is dangerously exothermic and takes place in explosive violence because of the great affinity of fluorine for hydrogen. It brings about the rupture of C-C and C-H bonds leasing to the mixture of the product.
CH4 + 2 F2 → C + 4HF
However, the controlled fluorination of alkane to form fluoro derivatives can be carried out by diluting with inert gas and an apparatus designed to carry away the produced heat.
The iodine is generally unreactive.
Relative reactivities of alkane toward halogenation reaction
The method of competition is used to determine the relative reactivities of different compounds to the same reagent. Under identical reaction conditions, an equimolar amount of two compounds to be compared is treated with a limited amount of a specific reagent. Due to the scarcity of reagents, both compounds compete with one another. It is demonstrated that more reactive compounds consume more reagents after the reaction is completed.
For example, the chlorination of methane and ethane
If an equimolar amount of these alkanes is allowed to react with a small amount of chlorine in presence of light at 25o C, about 400 times as much ethyl radical as methyl radical is obtained. This shows ethane is 400 times as reactive than methane towards chlorination.
CH4 + Cl2 → CH3Cl (1)
C2H6+ Cl2→C2H5Cl (400)
This result can be attributed to two factors below:
1. Probability factor:
Ethane has six hydrogen atoms that can be replaced by chlorine, whereas methane has only four hydrogen atoms. As a result, the chances of forming ethyl chloride and methyl chloride will be 6:4 or 3: 2
2. Relative reactivities of different classes of the hydrogen atom
This determines the proportion of products formed during the halogenation of methane and ethane (or other alkanes). This is dependent on the relative stabilities of the alkyl radicals formed in the step that controls the overall halogenation reaction.
In the case of methane and ethane, this may be written as:
CH4 + Cl. → CH3. (Methyl radical) + HCl
CH3 – CH3 → CH3 – CH2. (Ethyl radical) + HCl
The stability of ethyl radical is more than the stability of methyl radical. As a result, the transition state involved in the formation of ethyl radicals from ethane is more stable than the transition state involved in the formation of methyl radicals from methane. As the transition state of ethyl radical requires less Eact than the transition state of methyl radical, ethyl radical forms faster than methyl radical. As a result, the chlorination of ethane occurs more easily than methane, and the resulting mixture contains a much higher proportion of ethyl chloride than methyl chloride.
Reactivity and selectivity of halogens
When an alkane contains more than one type of hydrogen atom, the isomeric product can be formed from a single alkane. Ethane gives only one haloethane product because it contains only primary hydrogen while propane, butane, and isobutane can yield two isomeric products because they contain two types of halogens.
CH3 – CH3 + HCl → CH3 – CH2 – Cl + HCl
CH3 – CH2 – CH3 + HCl → CH3 – CH2 – CH2 – Cl+ CH3 – CHCl– CH3
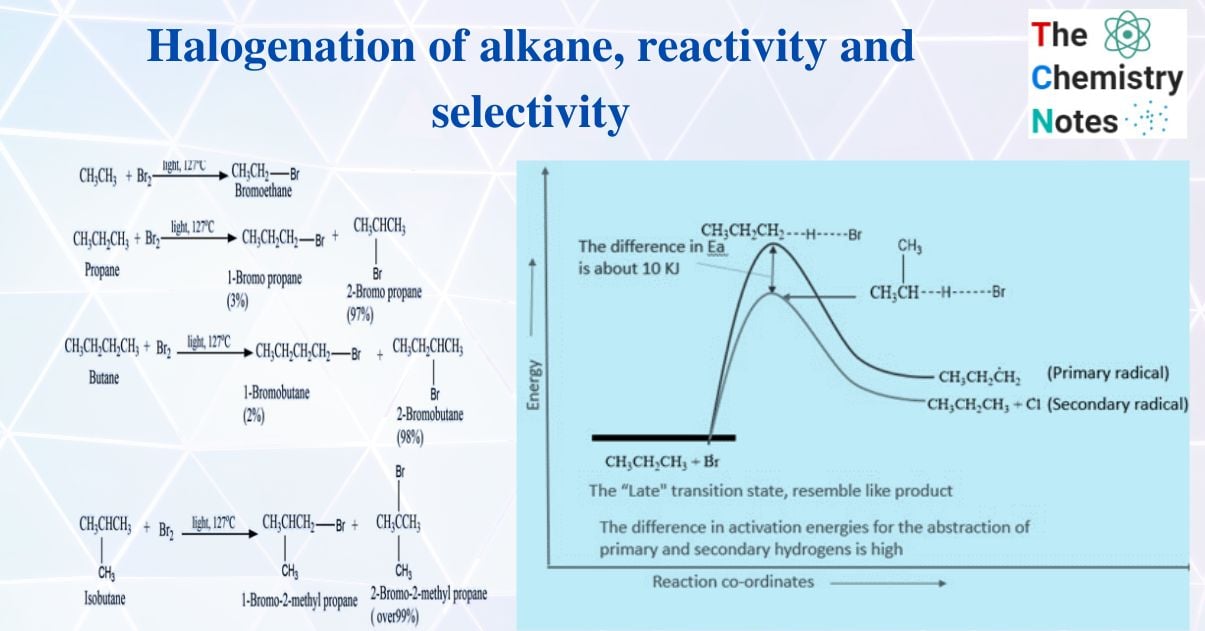
Chlorination of alkane
Experiments have shown that all hydrogen atoms of methane are susceptible to replacement. Chlorination of methane gives chloromethane only at controlled reaction conditions. It shows that all hydrogens in methane are chemically equivalent. Further chlorination of chloromethane (CH3Cl) leads to polychlorinated products where carbon tetrachloride is the final product.
Bright sunlight is the most energetic and causes the instantaneous combustion of alkane, so bright sunlight is not suitable for chlorination.
CH4 + 2 Cl2 → C + 4 HCl
The addition of tetraethyl lead (C2H5)4 Pb initiates the chlorination of methane in dark at 150O due to the formation of C2H5 radicals.
(C2H5)4 Pb → 4 C2H5. + Pb
The ethyl radical then reacts with chlorine molecules forming ethyl chloride and chlorine free radical.
Cl – Cl + C2H5. → C2H5 – Cl + Cl.
The chlorine freeradical thus formed brings about the chlorination of methane.
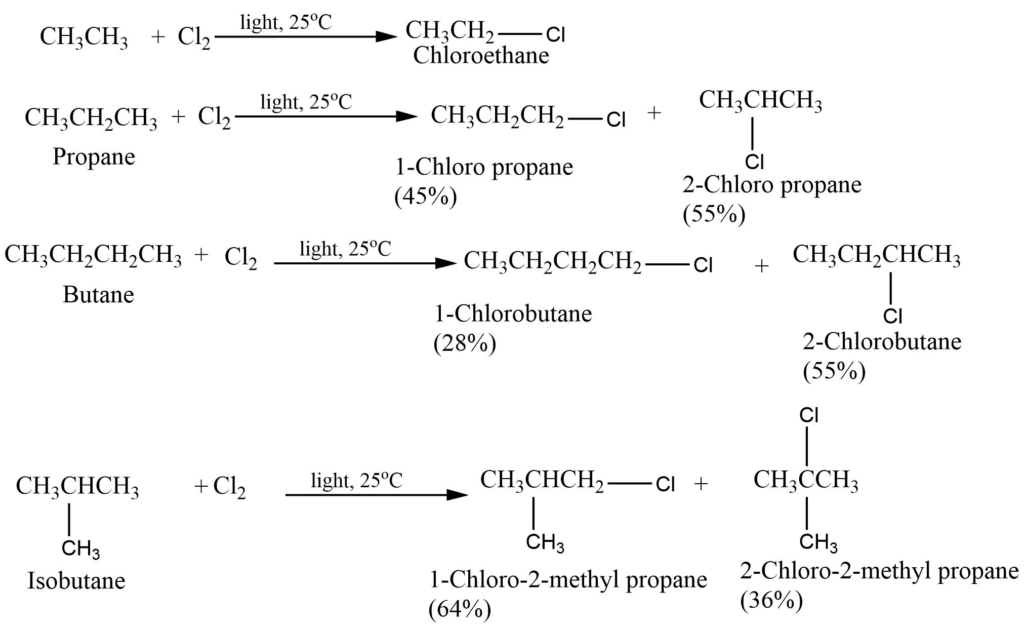
Bromination of alkane
Bromination of alkane also takes place in a similar manner but less readily. Bromine is less reactive and more selective than chlorine.
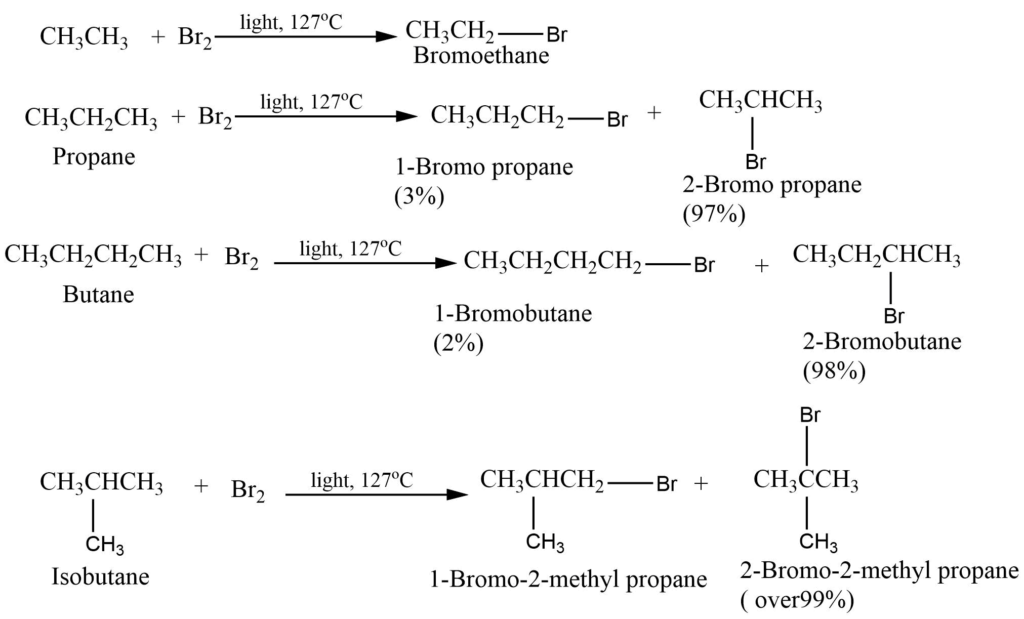
Chlorination of alkane gives isomeric chloroalkane in which no isomer greatly predominates but in bromination one isomer may predominate to such an extent as to be almost the only product making up to 97 – 99 % of the mixture.
It can be explained on the basis of reactivity and selectivity. Chlorine is more reactive but less selective in its attack. Whereas bromine is less reactive but there is a high degree of selectivity to which hydrogen atoms are to be abstracted.
Selectivity of bromine
The higher selectivity of bromine can be explained in terms of transition state theory. Endothermic hydrogen abstraction by less reactive bromine atoms has a very high Eact.The transition state resembles the product more than it does the reactants. The transition state is reached late in the reaction process after the alkyl group has developed a considerable radical character.
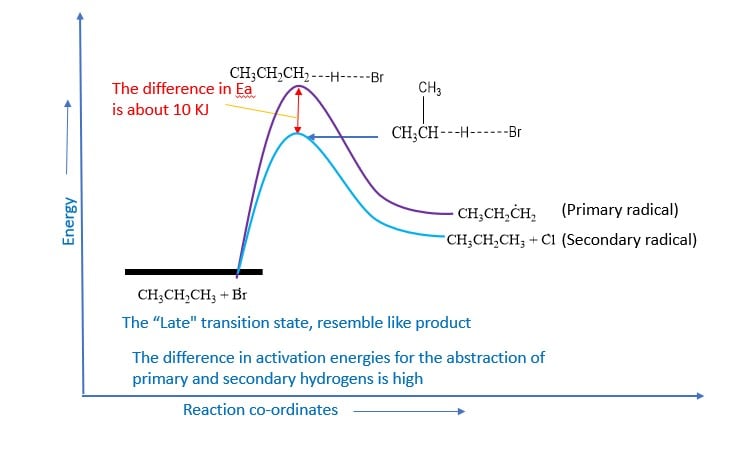
On the other hand, hydrogen abstraction by highly reactive chlorine atoms is exothermic and has a low Eact. The transition state is more like the reactant than the product.The transition state resembles the reactant more than it does the product. The transition state is reached early in the reaction process and the carbon-hydrogen bond is slightly stretched. The alkyl group has developed very little free radical character.
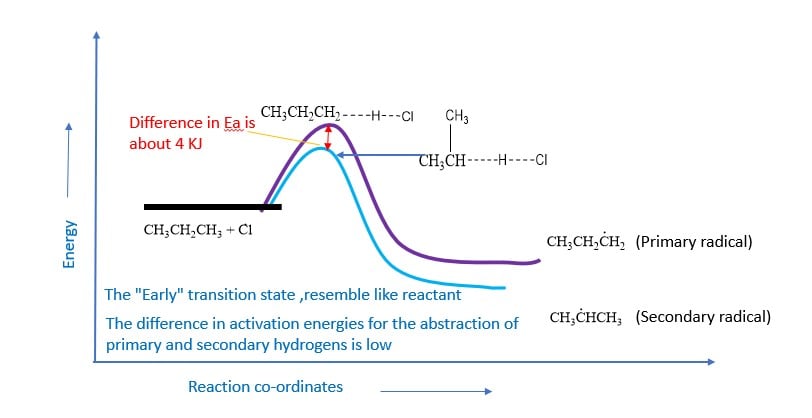
Flourination and iodination
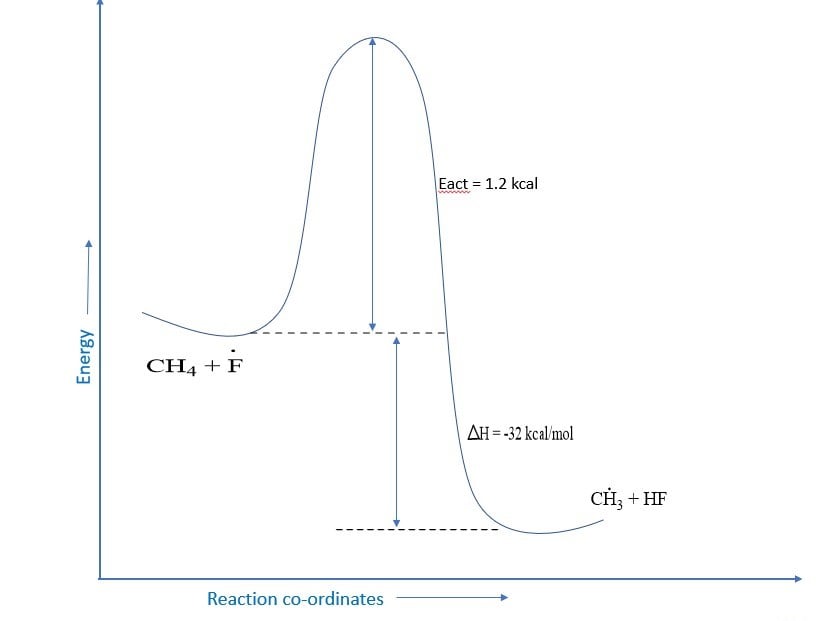
Fluorine, being much more reactive than chlorine, is even less selective than chlorine Because the activation energy for the abstraction of any type of hydrogen by a fluorine atom is low, the rate at which 1O, 2O, or 3O hydrogen reacts with fluorine differs very little. Reactions of alkanes with fluorine give the distribution of a product that we would expect if all hydrogens of alkanes were equally reactive.
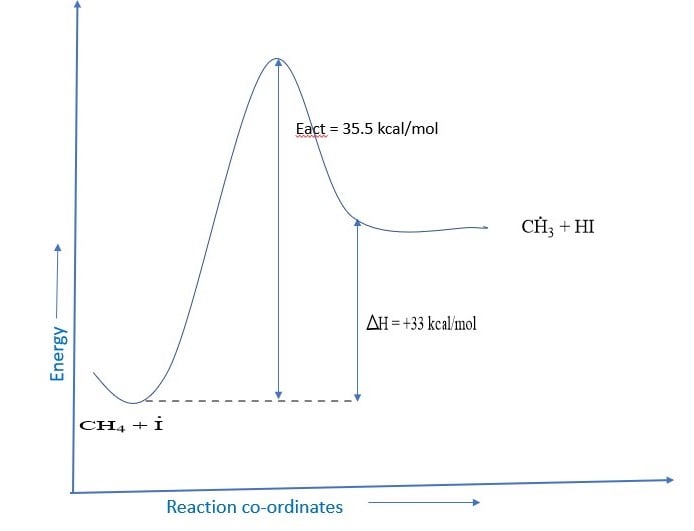
Iodination of an alkane is very slow and reversible because iodine is the least reactive halogen and HI produced in the first step reduces the alkyl halide back to the parent.
R – H + I2 ⇌ R – I + HI
So direct iodination of an alkane is not a feasible reaction. However, HI can be removed with the help of HNO3 or HIO3. iododerivatives of alkane can be obtained by direct iodination.
5 HI + HIO3 → 3 I2 +2 H2O
Orientation of halogenation reaction
Let’s take an example, chlorination of propane. The chlorination reaction takes place at room temperature in presence of sunlight to give n-propyl chloride and isopropyl chloride in a ratio of 45: 55.
The relative amounts of n-propyl and isopropyl chloride obtained depend upon the relative rates at which n-propyl radicals and isopropyl radicals are formed as the rate-determining step by the attack of chloride radical on propane at the proper site.
The rates at which the two propyl radicals are formed depend upon the following three factors:
1. Collision frequency:
The collision frequency must be the same for the two reactions because both involve the collision of the same particles viz, a propane molecule and a chlorine atom.
2. Probability factor
Since there are six primary hydrogens and only two secondary hydrogens in each molecule of propane, the probability of abstraction of primary hydrogen as compared to the secondary hydrogen should be in the ratio of 6: 2, or 3: 1.
If we consider the probability factor and collision frequency only, the chlorination of propane would be expected to yield n-propyl chloride and isopropyl chloride in the ratio of 3: 1. But in actual practice, the two chlorides are formed in roughly equal amounts that are, in the ratio of 1: 1 or 3: 3.
3. Energy factor
The probability of the formation of isopropyl chloride is about three times as great as that of the formation of n- propyl chloride. This means that collisions with secondary hydrogens are about three times more successful than a collision with primary hydrogens. If our assumption about the probability factor is correct, this means that Eact is less for the abstraction of secondary hydrogen than for the abstraction of primary hydrogen.
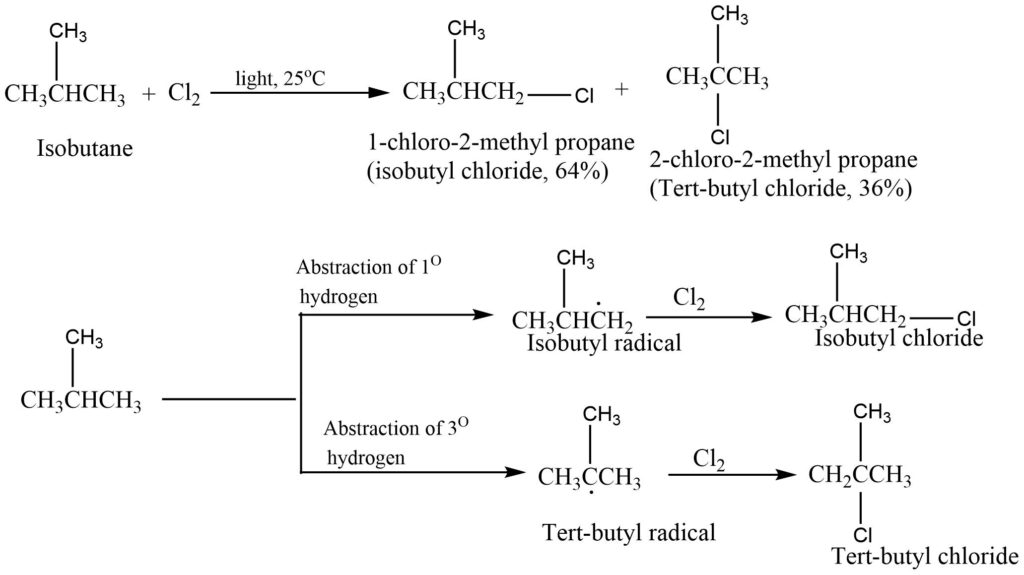
The isopropyl radical is a secondary radical while the n-propyl radial is the primary radical and the stability of radicals is in the order of: Tert. Radical > Sec. radical > Pri. Radical.
Hence the orientation in the chlorination of propane is determined by the energy factor i.e., the Eact (energy of activation) for the abstraction of secondary and primary hydrogen in propane.
Suggested videos:
References
- Smith M. B. & March J. (2007). March’s advanced organic chemistry : reactions mechanisms and structure (6. ed.). Wiley-Interscience.
- Bahl A. & Bahl B. S. (2006). A textbook of organic chemistry (for b. sc. students) (18th rev. & enlarged ed. 1st multicolour illustrative). S. Chand.
- https://byjus.com/chemistry/halogenation-of-alkanes/#:~:text=Halogenation%20of%20Alkanes-,What%20is%20Halogenation%20of%20Alkanes%3F,which%20reactions%20can%20take%20place.
- https://kpu.pressbooks.pub/organicchemistry/chapter/9-2-halogenation-reaction-of-alkanes/
- https://www.chemistrysteps.com/selectivity-in-radical-halogenation/
- https://www.masterorganicchemistry.com/2013/10/31/selectivity-in-free-radical-reactions-bromine-vs chlorine/#:~:text=In%20chlorination%2C%20the%20reaction%20is,transition%20state%20resembles%20the%20products.
- https://crab.rutgers.edu/~alroche/Ch04.pdf
- http://icvcollege.edu.in/sites/default/files/Radical%20Reactions.pdf?fbclid=IwAR12knOEQ3rQ3ai6gSZv6UqUI-L9-V59TF5QWgDEZ5i24dTWVQlU954q2H8
