Aldehyde and ketones are commonly known as carbonyl compounds. Both aldehyde and ketone consist of a carbon-oxygen double bond as a functional group. Which is known as the carbonyl group.
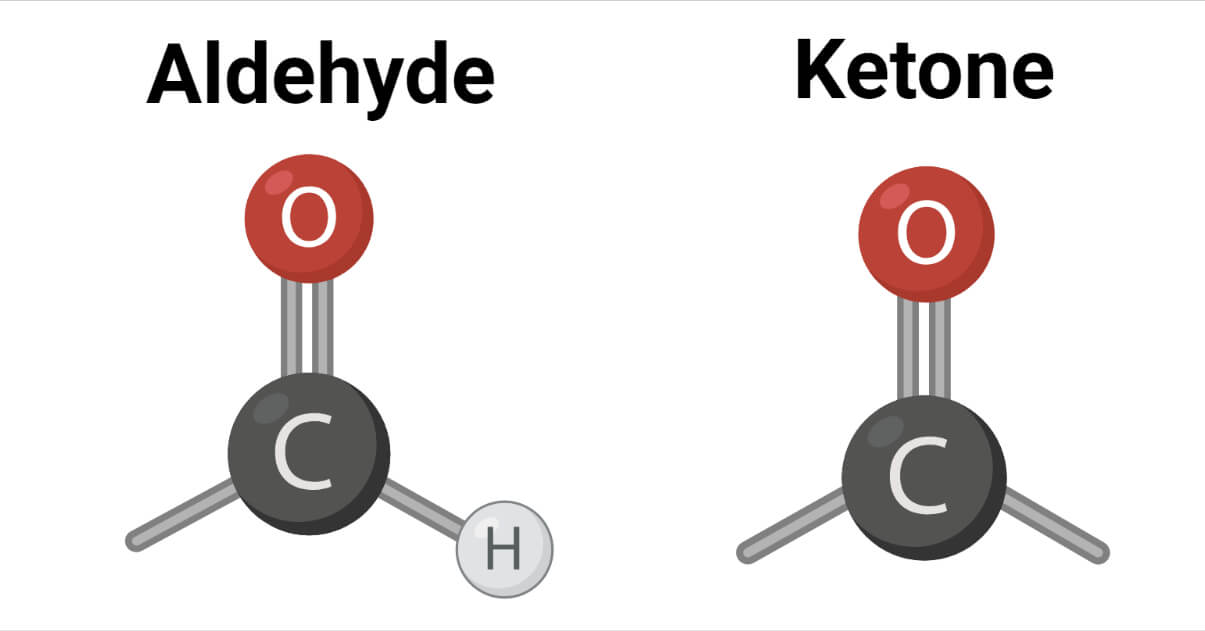
The carbonyl group in an aldehyde is bonded to one alkyl group and one hydrogen, whereas the carbonyl group in a ketone is bonded to both alkyl groups. Alkyl groups in ketone may be the same or different. In formaldehyde, the carbonyl group is bonded to two hydrogen atoms.
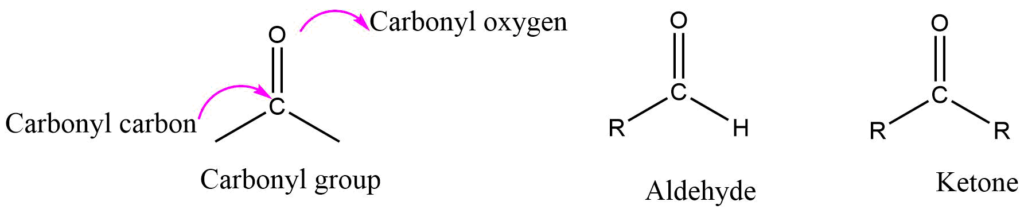
Structure of Carbonyl Group (Aldehyde and Ketone)
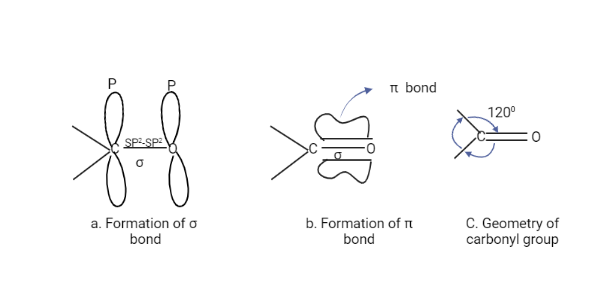
The Carbonyl group consists of one σ and one π bond. The overlap of the SP2 orbitals of carbon and oxygen forms the sigma bond, whereas the overlap of unhybridized P orbitals of carbon and oxygen forms the pi bond. Because the carbonyl carbon is SP2 hybridized, all of the atoms attached to it are in the same plane, with a bond angle of about 120o between them.
Nomenclature of Aldehyde and Ketone
There are two systems of naming aldehyde and ketones.
1. Common name system
Common names of aldehyde are obtained from the name of the corresponding carboxylic acid by replacing the ending -ic of the acid name with -aldehyde.
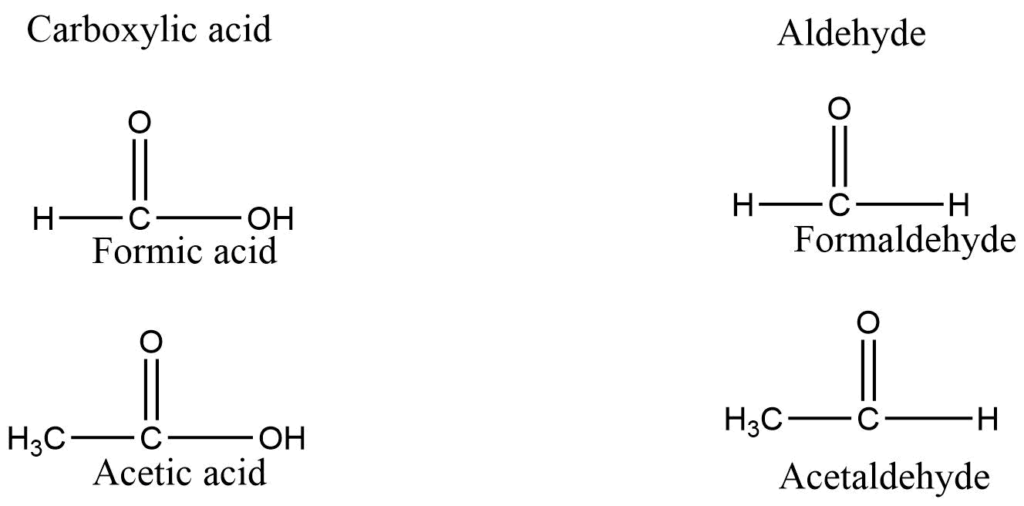
Common names for ketone are obtained by naming the alkyl groups attached to the carbonyl group and adding the word ketone.
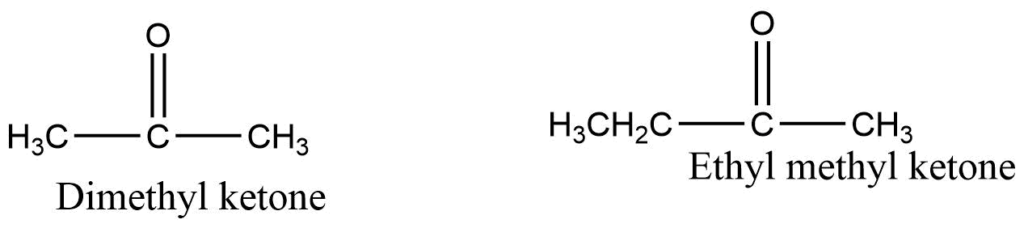
2. IUPAC system
IUPAC names for an aldehyde are obtained by replacing the ending -e of the corresponding alkane with -al while the ending -e of the corresponding alkane is replaced with -one in the IUPAC names for ketones. The carbon atoms are numbered in such a way that the carbonyl atom has the lowest number.
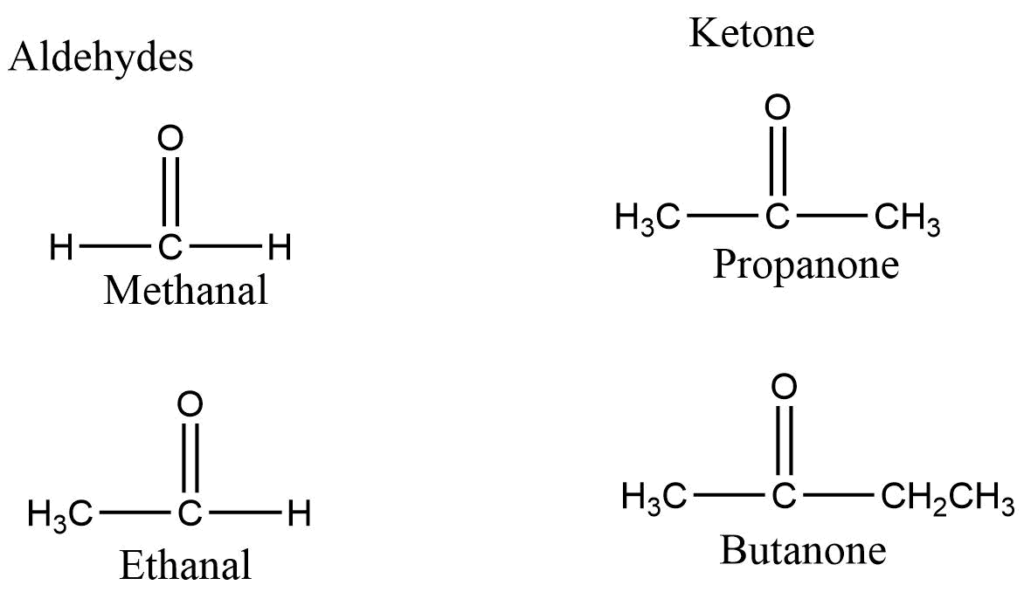
Methods of Preparation of Aldehyde
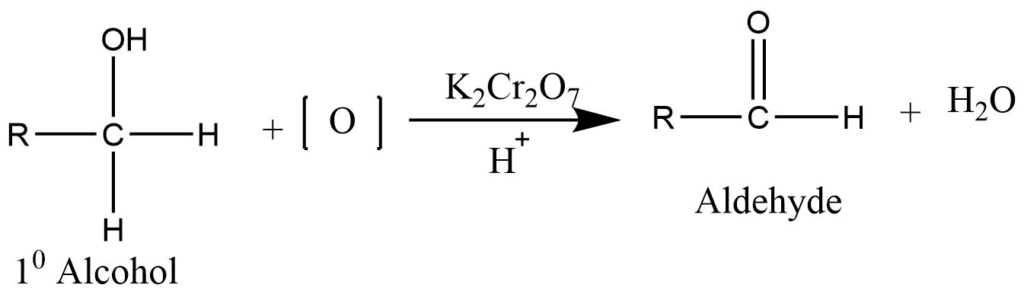
Oxidation of primary alcohol
Controlled oxidation of primary alcohol using the acidified solution of potassium dichromate or permanganate produces aldehyde.
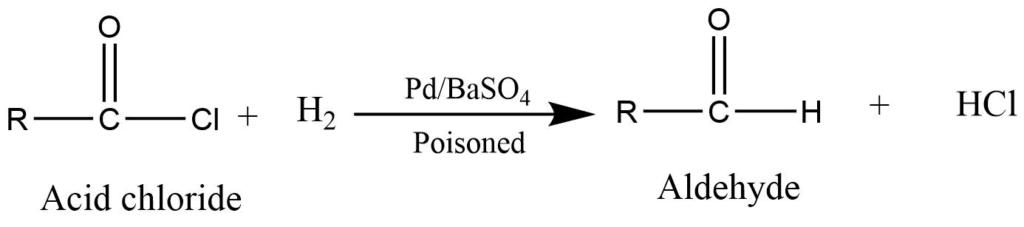
Reduction of acid chloride (Rosenmund Reaction)
Reduction of acid chloride by hydrogenation in presence of palladium poisoned by barium sulfate gives aldehyde.
Oxo process
Alkene reacts with carbon monoxide and hydrogen at high temperatures and pressures in the presence of a cobalt carbonyl catalyst to produce aldehyde. This is an important method for the preparation of aldehydes industrially.
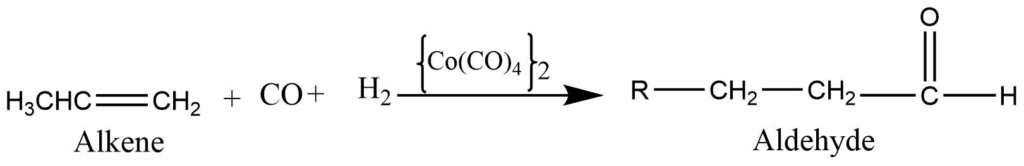
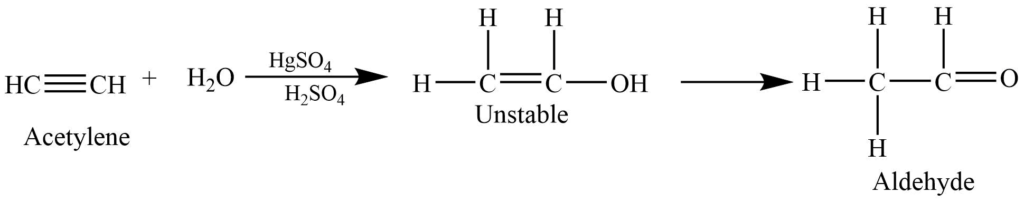
Hydration of alkynes
The addition of water to the alkynes in presence of mercuric sulfate and sulfuric acid forms an enol intermediate which rearranges to give acetaldehyde.
Methods of Preparation of Ketone
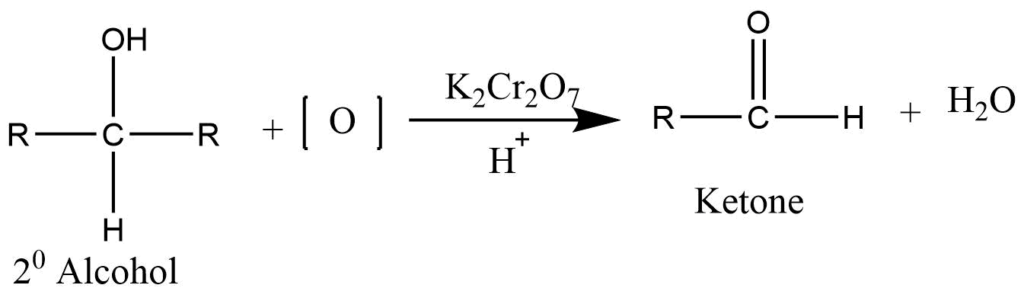
Oxidation of secondary alcohol
Controlled oxidation of secondary alcohol using the acidified solution of potassium dichromate or permanganate produces ketone.
Catalytic decomposition of acids
Vapors of suitable carboxylic acid on passing over MnO or ThO2 produce the symmetrical ketone.
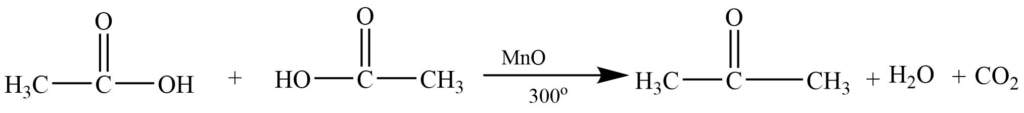
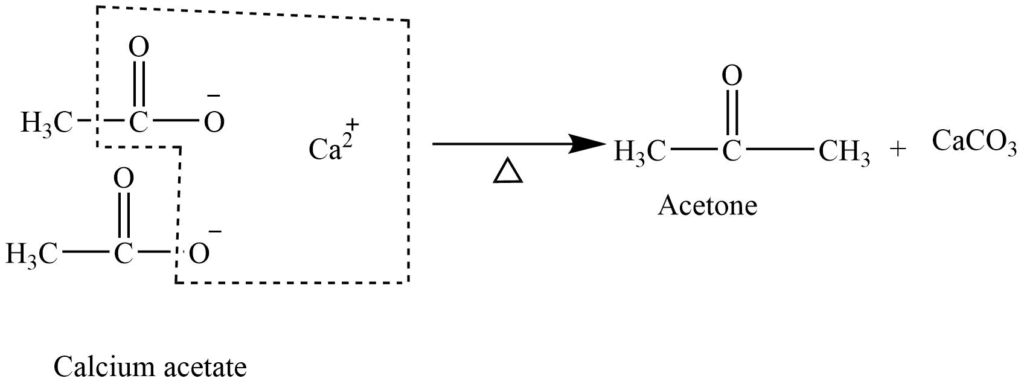
Pyrolysis of calcium salts of acids
The calcium salt of acids on heating at 4000C gives symmetrical ketone.
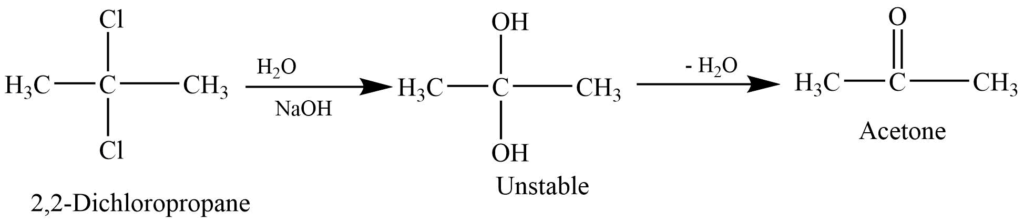
Alkaline hydrolysis of gem dihalides
On alkaline hydrolysis of the gem-dihalides containing two halogen groups at the nonterminal hydrogen gives a ketone.
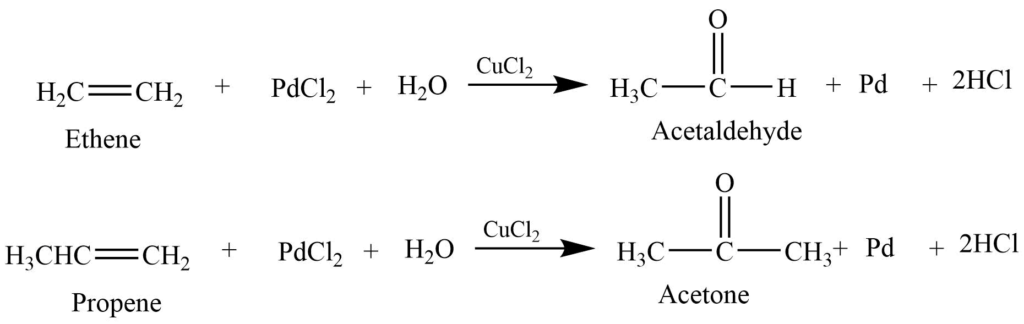
Wacker process
Alkene reacts with an acidified aqueous solution of palladium chloride and cupric chloride to produce aldehyde or ketone. The type of alkene determines the nature of the product.
Physical Properties of Aldehyde and Ketone
- Formaldehyde is gas at room temperature. Acetaldehyde boils at 20oC while other lower aldehyde and ketones are colorless liquids.
- Lower aldehydes possess an unpleasant smell while ketones have pleasant sweet odours.
- Aldehydes and ketones have a higher boiling point than alkanes but a lower boiling point than alcohols.
- Lower aldehydes and ketones are soluble in water. Solubility decreases as the number of hydrocarbons increases.
- Aldehyde and ketone have a lower density than water.
- Aldehyde and ketones show a C=O stretching absorption band in the 1665-1780 cm-1 region. -CHO group of aldehyde shows additional bands at 2700-2900cm-1 and 2820-2900 cm-1
Chemical Properties of Aldehyde and Ketone
Nucleophilic addition reactions
The Carbonyl group of aldehyde and ketones, is highly polar in nature. During the reaction, electron-rich nucleophiles attack the positively charged nucleophiles while oxygen is attacked by electron-deficient electrophiles.
Addition reactions
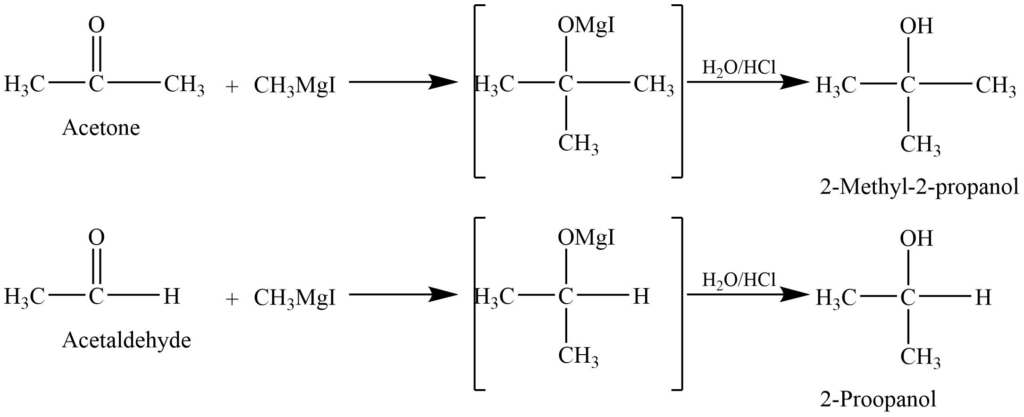
- Addition of Grignard reagent: Reaction of aldehyde and ketone with the Grignard reagent to give addition product which on hydrolysis with dilute acid gives alcohol.
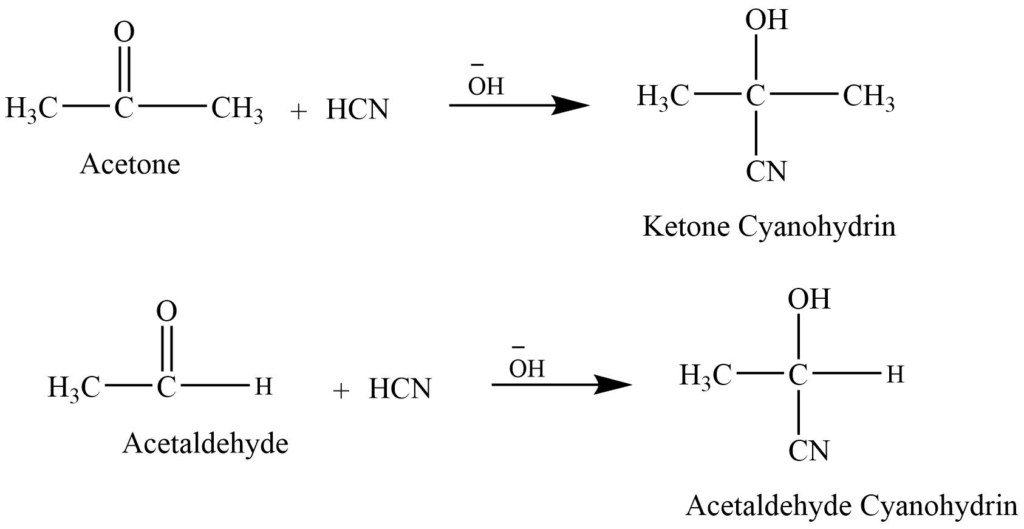
- Addition of hydrogen cyanide: Aldehyde and ketone react with hydrogen cyanide in presence of base catalyst to give cyanohydrins.
- Addition of ammonia: Aldehyde reacts with ammonia to give aldehyde ammonia . Aldehyde ammonia on heating with dilute acids regenerates aldehyde so this reaction is also used for the purification of aldehydes. (Formaldehyde and acetone don’t give an addition compound with ammonia.

Reaction with ammonia derivatives
Some ammonia derivatives react with aldehyde and ketone to give compounds containing a carbon-nitrogen double bond.
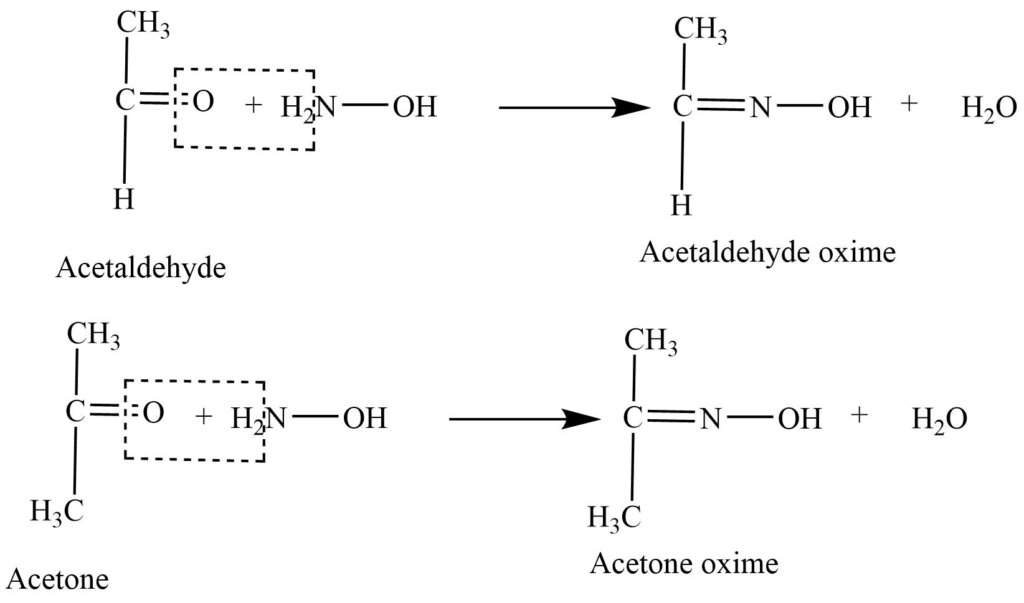
- Reaction with hydroxylamine: Reaction of aldehyde and ketone with hydroxylamine (NH2OH) gives oxime.
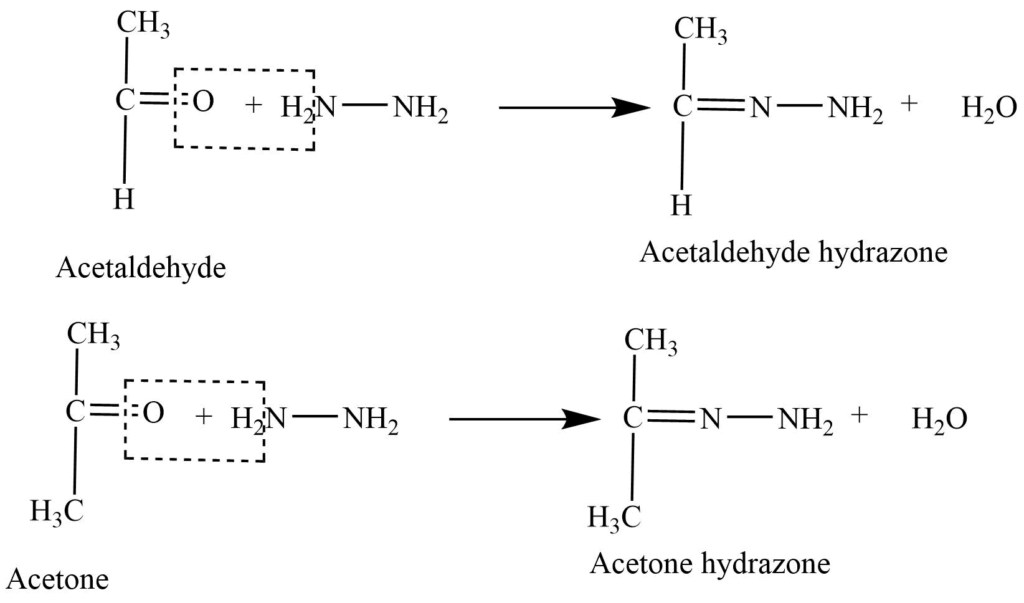
- Reaction with hydrazine: Aldehyde and ketones react with hydrazine (NH2-NH2) to produce hydrazone.
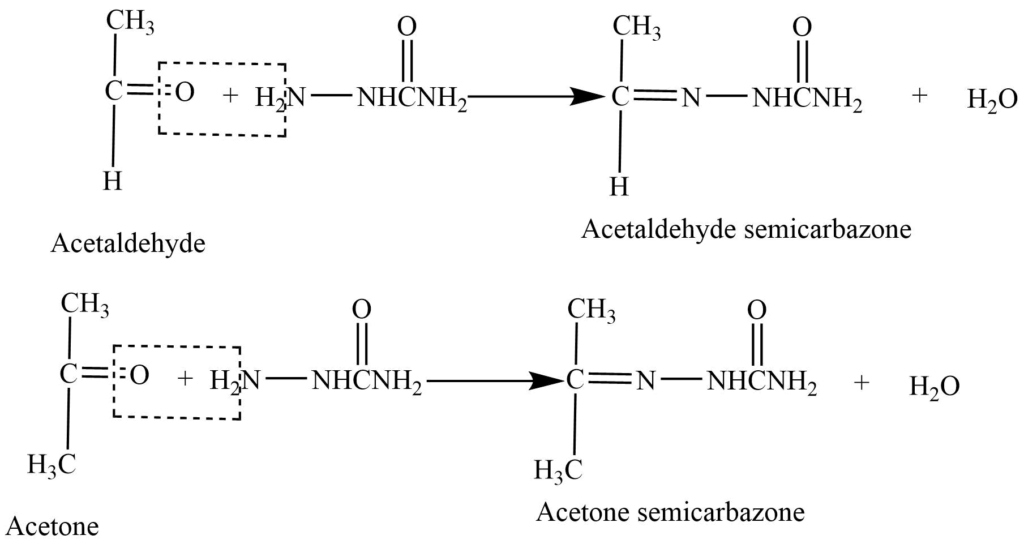
- Reaction with semicarbazide: Aldehyde and ketones on reaction with semicarbazide gives semicarbazones.
Condensation reaction
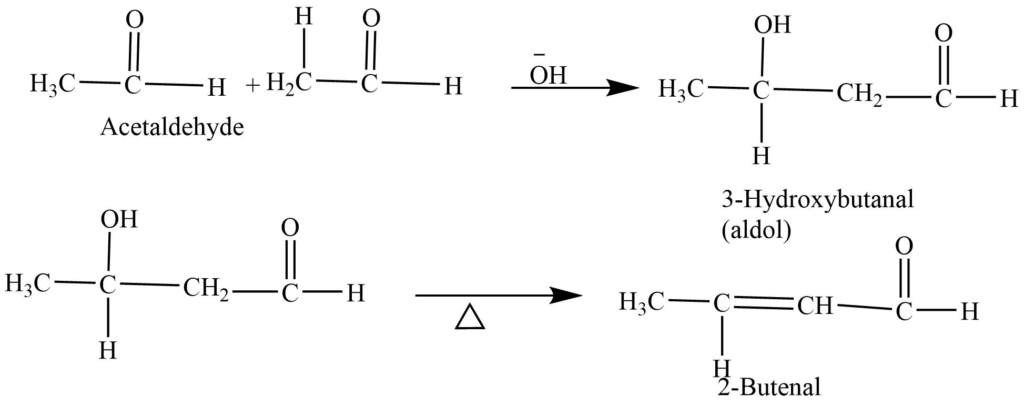
- Aldol condensation: In the presence of a base, aldehydes containing alpha hydrogen undergo a self-addition reaction to form aldol. Aldol on treatment with dilute acid or on heating dehydrated to give α – β unsaturated product.
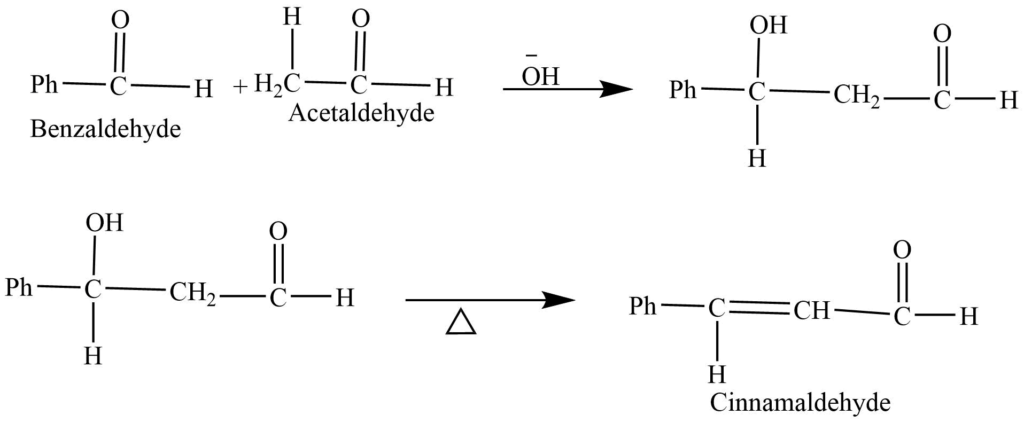
- Mixed aldol condensation: The mixed aldol condensation reaction is the reaction of two different aldehydes or ketones, (one of which contains alpha hydrogen) in the presence of a base.
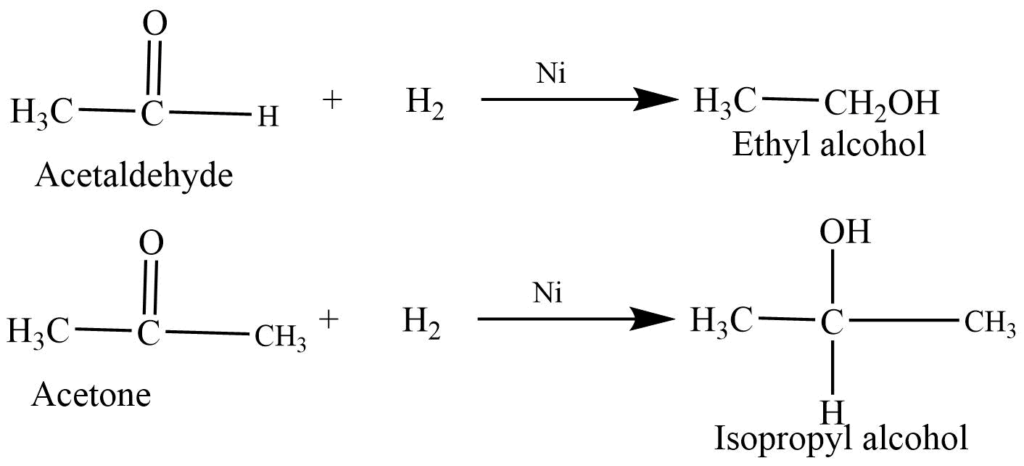
Reduction Reaction
- Reduction to alcohols: Aldehyde and ketones on reaction with hydrogen and Ni or Pt catalyst reduce to alcohols.
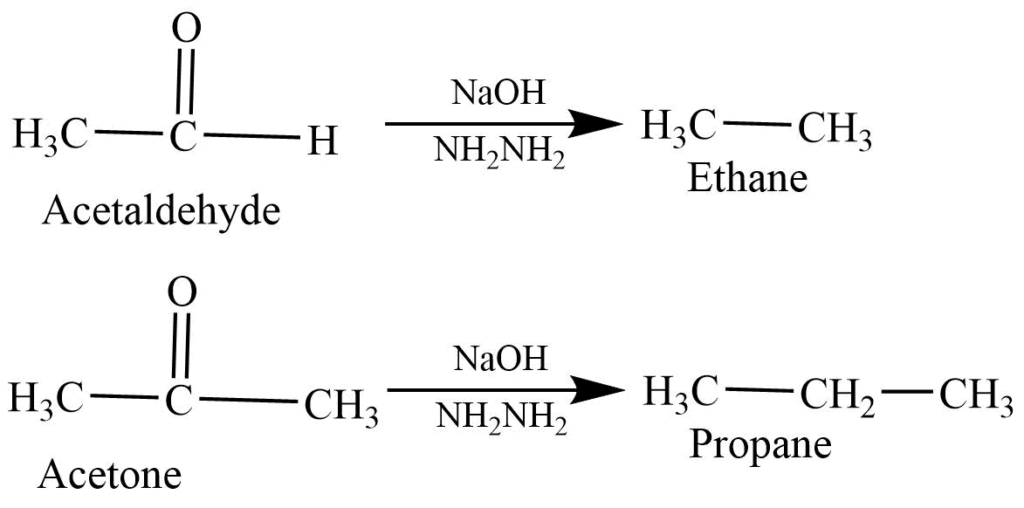
- Wolf – Kishner reaction: Aldehyde and ketone react with a basic solution of hydrazine to form alkane.
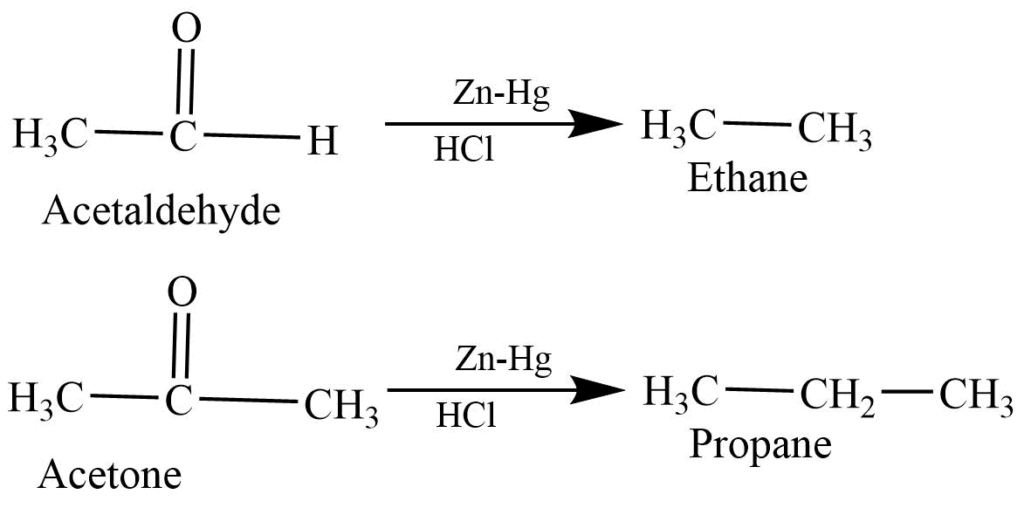
- Clemmensen reaction: The reaction of aldehyde and ketone with Zn-Hg in Hcl produces alkane.
Oxidation Reaction
Oxidation of aldehyde: Oxidation of aldehyde with sodium or potassium dichromate in acidic medium forms carboxylic acid.
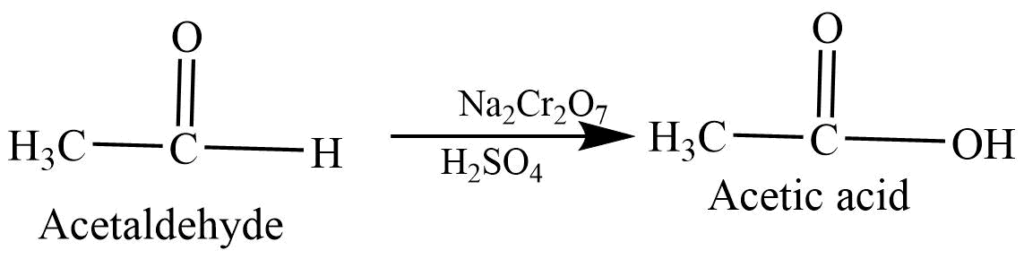
Oxidation of ketones: In the presence of a strong oxidizing agent such as alkaline KMnO4 or hot concentrated HNO3, a ketone can be oxidized to form two molecules of carboxylic acid with fewer carbons than the parent ketone.

Other Reactions
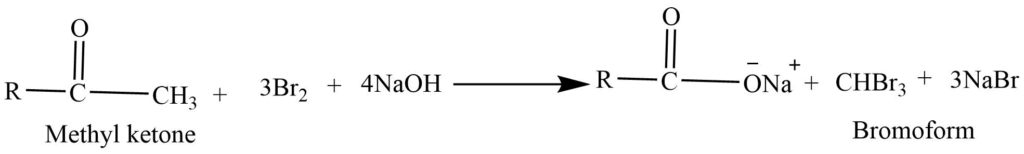
- Haloform reaction: In presence of base aldehyde and ketone reacts with halogens (Cl2, Br2 or I2) to form haloform.
- Cannizzaro reaction: Aldehydes without the alpha hydrogen when heated with concentrated NaOH undergoes a disproportionation reaction. In this reaction, one half of aldehyde oxidized to carboxylic acid while another half reduced to form alcohol.
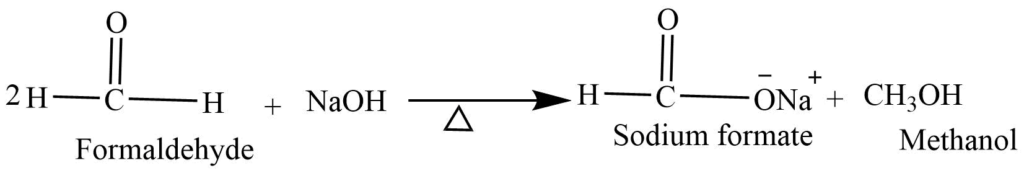
Uses of Aldehyde and Ketone
- Aldehydes and ketones are used as starting materials for many organic synthesis reactions.
- Formaldehyde is used to make glues, insecticides, and fungicides.
- Aldehydes can be used for tanning, and as a preservative.
- Ketones are used as a solvent in the production of paint, textiles, as well as in hydraulic fluids.
- Aldehydes can be used as perfumes and flavoring agents. It can also be used to make pharmaceuticals, plastics, and dyes as intermediates.
References
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- https://byjus.com/chemistry/aldehydes-ketones/
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/aldket1.htm
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Aldehydes_and_Ketones/Nomenclature_of_Aldehydes_and_Ketones
- https://www.vedantu.com/chemistry/physical-properties-of-aldehydes-and-ketones
- https://byjus.com/chemistry/cannizzaro-reaction-mechanism/
- https://byjus.com/chemistry/aldol-condensation/
- https://www.masterorganicchemistry.com/2018/08/27/the-wolff-kishner-clemmensen-and-other-sidechain-reductions/
- https://www.britannica.com/science/aldehyde/Uses-of-aldehydes
- https://www.geeksforgeeks.org/uses-of-aldehydes-and-ketones/
