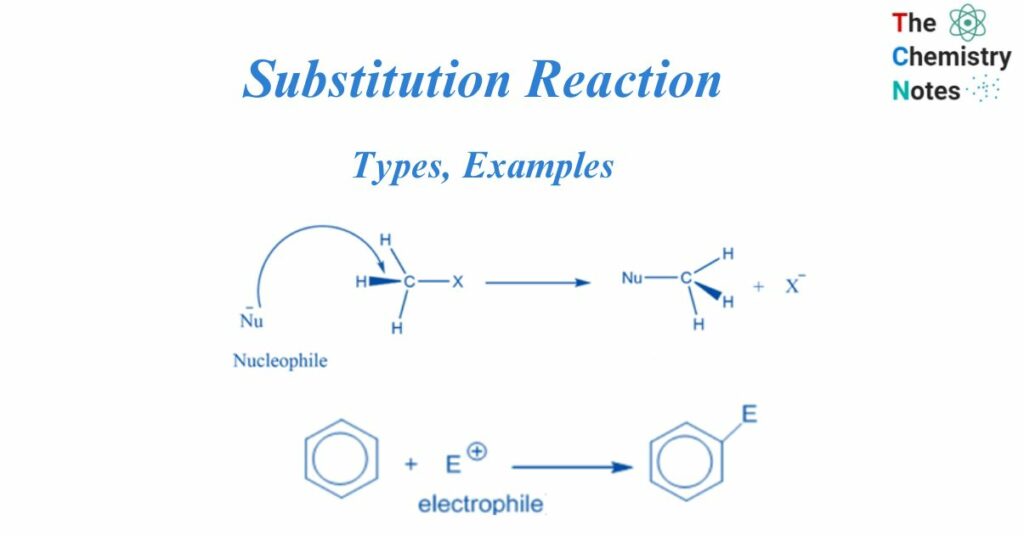
A substitution reaction is any chemical process that replaces one atom, ion, or group in a molecule with another. Substitution reactions in organic chemistry are characterized as electrophilic or nucleophilic based on a variety of parameters. These elements include the reagents, reactive intermediates, and substrates.
Substitutions in organic chemistry involve the replacement of one functional group in an organic molecule with another functional group.
CH3CH2OH + HI → CH3CH2I + H2O
Nucleophilic substitution reaction
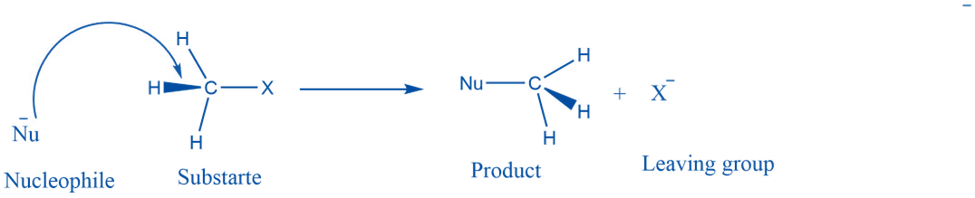
A nucleophilic substitution reaction is a type of organic reaction in which one nucleophile substitutes another. The group that takes an electron pair and is displaced from the carbon is known as the “leaving group,” and the molecule on which substitution occurs is known as the “substrate.” The departing group exits as a neutral molecule or anion.
They are a type of reaction in which one nucleophile attacks a positively charged electrophile to replace another nucleophile.
This reaction takes place by the attack of the nucleophile on the electron-deficient center of the substrate to form the product.
Nucleophile:
A nucleophile is a chemical that contributes an electron pair to the formation of a covalent bond. A nucleophile is often negatively charged or neutral, with a single pair of donatable electrons. In chemistry, a nucleophile is an atom or molecule that seeks a positive center in a chemical reaction, such as the nucleus of an atom, since the nucleophile possesses an electron pair accessible for bonding. Some examples include OR-, H2O, -OMe, or -OtBu, Cl-, and Br-. The electron-rich species is a nucleophile in general.
Types of nucleophilic substitution reactions
SN1 reaction
It is also known as an unimolecular nucleophilic substitution reaction. Unimolecular nucleophilic substitution consists of a two-step reaction mechanism. In which the formation of carbocation takes place before the nucleophile approaches the substrate. The mechanism is independent of the incoming nucleophile’s nucleophilicity but dependent on the leaving capacity of the leaving group. So, the rate of SN1 reaction depends on the concentration of substrate only.
Commonly the reaction of a tertiary or secondary alkyl halide with secondary or tertiary alcohols under strongly acidic or strongly basic conditions occurs via an SN1 reaction.
SN2 reaction
The bimolecular nucleophilic substitution reaction (SN2 reaction) is a one-step reaction that involves the transition state. The attack of a nucleophile on the substrate and the departure of the leaving group occur simultaneously in this reaction. Since both the substrate and the nucleophile are present in the transition state, the reaction is bimolecular.
Electrophilic substitution reaction
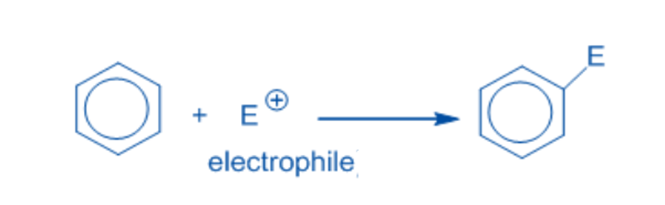
An electrophilic substitution reaction is a chemical process in which an electrophile replaces the functional group connected to a molecule. The displaced functional group is usually a hydrogen atom.
The primary distinction between nucleophilic and electrophilic substitution reactions is that the former requires the displacement of a leaving group by a nucleophile, whereas the latter involves the displacement of a functional group by an electrophile.
Electrophiles:
Electrophiles are electron-deficient chemical species that can accept an electron pair from an electron-rich species. Some example includes, H+, SO3H+, NO+, NO2+, X+, R+, C6H5N2+.
Types of electrophilic substitution reaction
There are two types of electrophilic substitution reaction processes. They are:
- Electrophilic aliphatic substitution reaction
- Electrophilic Aromatic substitution reaction
Electrophilic aliphatic substitution reaction
‘Electrophilic aliphatic substitution reactions’ occur when an electrophile displaces a functional group in an aliphatic molecule. In these reactions, the electrophile attacks the aliphatic molecule, resulting in a 180° inversion. The electrophilic substitution in aliphatic compounds is identical to the nucleophilic substitution in aliphatic compounds, except that an electrophile replaces a functional group rather than a nucleophile. The two basic electrophilic substitutions are SE2 (substitution electrophilic bimolecular) and SEi (substitution electrophilic internal) processes.
Electrophilic aromatic substitution reaction
An aromatic compound undergoes an electrophilic substitution reaction when the aromatic ring is substituted or displaced by an electrophile. In such reactions, the aromaticity of molecules is retained. Examples of electrophilic aromatic substitution reactions include sulfonation, Friedel-Crafts reactions, and nitration in aromatic compounds.
References
- https://www.britannica.com/science/substitution-reaction.
- https://www.vedantu.com/chemistry/substitution-reaction.
- March, J. (1992). Advanced organic chemistry: Reactions, mechanisms, and structure. New York: John Wiley & Sons.
- Peter sykees, A Guide Book to Mechanism in Organic Chemistry, (6th edition) Pearson.
