
Bond cleavage is the dissolution of a chemical bond (usually a covalent bond). Covalent bond fission, or covalent bond breaking, can occur in two ways: i.e., Homolytic bond cleavage and Heterolytic bond cleavage.
Homolytic bond cleavage
Homolysis, also known as homolytic bond fission, takes place when a covalent bond between two atoms is broken, allowing each atom to take one of the bonding pairs of electrons. Homolytic bond fission, which occurs in the presence of heat, light, or organic peroxides, is the most prevalent method of bond fission in the vapor phase. The end products of hemolysis are free radicals, which are electrically neutral and have one unpaired (odd) electron.
Free radicals are extremely reactive due to this electron’s affinity to become connected as quickly as feasible. The reactions that occur as a result of free radical intermediate generation are quite rapid.
Because the electron pair divides evenly to each portion, both products contain a single electron. A radical (or free radical) is a species that comprises one or more single electrons. Homolytic cleavage results in the formation of radicals. The arrow with a sing-barb (like a fishhook) is used to demonstrate homolytic cleavage, which is a single electron transfer.
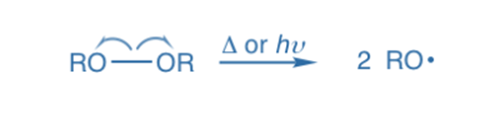
Heterolytic bond cleavage
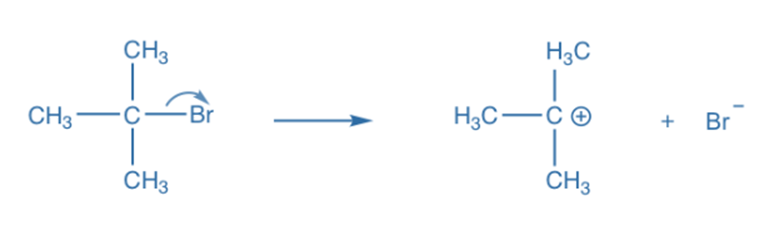
Heterolysis, also known as heterolytic bond fission, occurs when the covalent bond between two atoms is broken in such a way that one of the atoms receives both of the bonding pair of electrons. Ions are formed when the more electronegative atom accepts both electron bonding pairs. Heterolysis occurs most frequently in polar compounds and polar solvents. The rates of heterolytic fission reactions are predictable. In this type of covalent bond fission, the shared pair of electrons remains with one of the atoms. Carbocation and carbonation are formed as a result of heterolytic fission.
It should be emphasized that the positively charged product of a neutral molecule’s heterolytic fission, known as the cation, is the chemical species that did not retain any of the bonded electrons after the bond fission. The negatively charged result of heterolysis, on the other hand (also known as the anion), is the chemical entity that retains both bound electrons after the bond fission process.
The energy required to cleave a covalent bond via heterolytic cleavage is known as the heterolytic bond dissociation energy. The H-Cl bond is polar because chlorine is more electronegative than hydrogen. The bonding electrons of the H-Cl bond are more attracted to chlorine than hydrogen. During heterolytic cleavage, there is the formation of a proton and a chloride ion.
In an SN1 reaction, for example, the leaving group Br departs with the electron pair to generate a Br- and carbocation intermediate. The bond breaks heterolytically during heterolytic bond cleavage. An arrow with double barbs is used to depict heterolytic cleavage or the transfer of an electron pair in particular.
Bond dissociation energy
The strength of a chemical bond between two species can be calculated using bond dissociation enthalpy. Even though it is frequently measured as the enthalpy change of the homolytic fission of the bond at absolute zero (0K), the bond dissociation energy of a chemical bond is generally defined as the enthalpy change of the homolytic fission of the bond at absolute zero (0K) (298K).
Some major components of the bond dissociation enthalpy idea are as follows:
- It refers to the amount of energy required to break a chemical bond between two species.
- It’s a method of determining the strength of a chemical bond.
- In diatomic molecules, its value equals the bond energy value.
- The highest bond dissociation enthalpy is thought to be found in silicon and fluorine.
- Bond dissociation energies of covalent bonds between atoms or molecules are said to be small.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- https://byjus.com/chemistry/homolytic-and-heterolytic-fission/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_I_(Liu)/09%3A_Free_Radical_Substitution_Reaction_of_Alkanes/9.01%3A_Homolytic_and_Heterolytic_Cleavage
- https://www.differencebetween.com/what-is-the-difference-between-homolysis-and-heterolysis/
