A carbocation is a name used to describe organic chemical entities with an electrical charge on a carbon atom. The carbocation has a positively charged carbon. In general, these carbon atoms react with various elements to provide a reactant for additional reactions, known as intermediates.
Julius Stieglitz first proposed the concept of carbocation in 1899. However, Wagner-Meerwein’s rearrangement study, proposed by German chemist Hans Meerwein in 1922, brought additional research and insights.
What are Carbocations?
In organic chemistry, carbocations are essentially chemical entities that have a positive charge on carbon but only have six valence electrons. It is also known as carbonium ion. Because carbocation carbon only has six electrons, it is electron deficient and hence behaves as an electrophile in chemical processes.
Preparation of carbocation
Heterolytic cleavage
When a neutrally charged molecule undergoes heterolytic fission, any bonded atom that retains a shared pair of electrons (the one with stronger electronegativity) obtains a negative charge, whereas the remaining atoms that do not retain electrons acquire a positive charge. This positively charged intermediate is referred to as a carbocation intermediate.
e.g
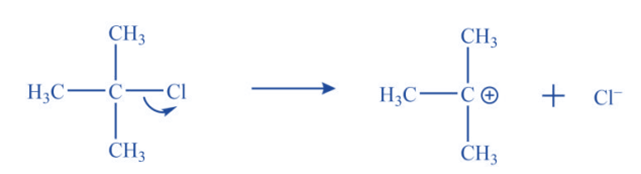
a. Ionization of alkyl halide in polar solvent
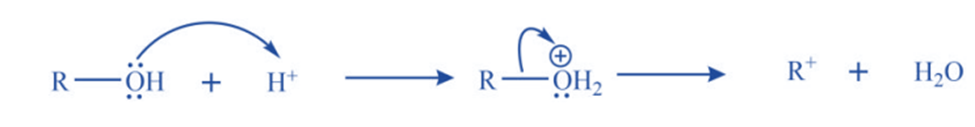
b. Protonation of alcohol followed by dehydration
c. Nitrous acid deamination of primary aliphatic amines
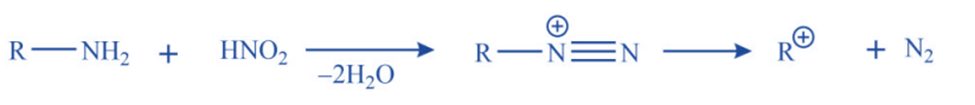
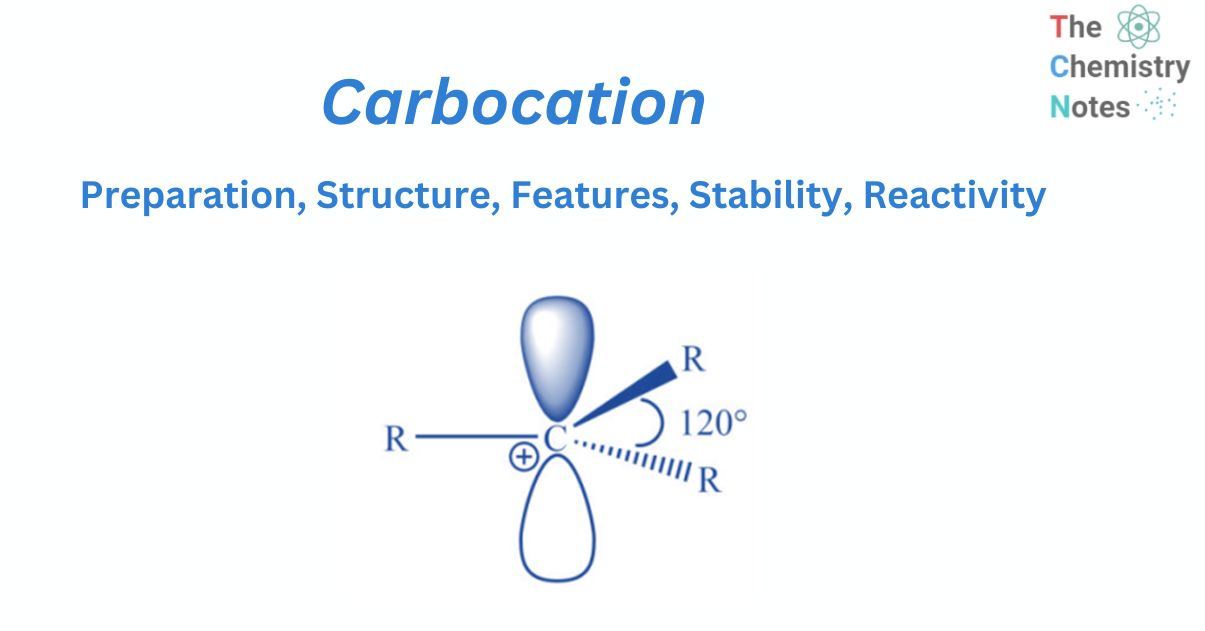
Structure of carbocation
It has been discovered experimentally that carbocations are trigonal planar around the carbon-bearing positive charge. Now that valence bond theory and molecular orbital theory can easily account for such structure, it is easier to understand the valence bond approach. The positively charged carbon is in sp2 hybridization, with three hybrid orbitals oriented at 1200 in a plane and an empty pz orbital perpendicular.
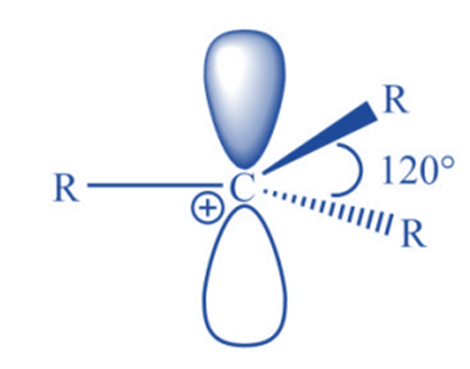
Features of carbocation
Carbocations have the following characteristics:
- There is a presence of Sp2 hybridization.
- Carbon atoms have triagonal planar geometry.
- It is extremely reactive, which means it is less stable.
- Because of insufficient electron pairing, carbocation exhibits paramagnetic behavior.
- It functions as an electrophile.
- It functions as Lewis Acid.
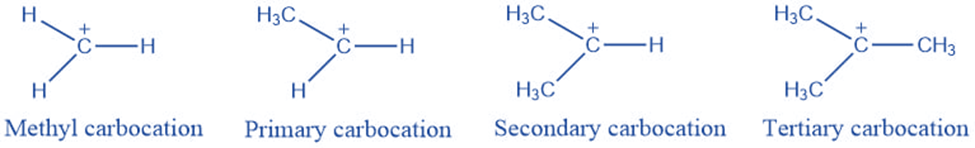
Types of carbocation
They are classified into the following categories based on the number of carbon groups connected to the positively charged carbon atom:
Methyl Carbocation: This is the least stable carbocation since no carbon group is connected to the carbon atom with a positive charge.
Primary carbocation: One carbon is linked to a positively charged carbon atom.
Secondary Carbocation: A positively charged carbon atom is joined to two other carbon atoms.
Tertiary Carbocation: It occurs when a positively charged carbon atom bonds with three other carbon atoms. It has the highest degree of stability.
Stability of carbocations
They are unstable intermediates that are extremely reactive. This means that they will immediately reorganize in order to construct more substantial structures. The stability of a carbocation is determined by its structure and the presence of substituents. The most stable carbocation is the tertiary carbocation, followed by the secondary carbocation. Its primary carbocation is the most unstable.
Carbocation stabilities can be calculated by measuring the amount of energy required to form the carbocation by dissociating the corresponding alkyl halide, with the tertiary alkyl halide separating much more easily than the primary or secondary, resulting in tri-substituted carbocations that are more stable than di- or mono-substituted ones. Polar solvents may rapidly stabilize the ions, giving in a lower dissociation enthalpy, but carbonation stability remains unchanged.
Factors affecting the stability of carbocation
Their stability is influenced by various factors. They are:
Resonance
The carbocation stability rises as the number of resonances increases. Furthermore, the more resonating structures there are, the more stable the carbocations are. The delocalization of the positive charge reduces electron loss even further, increasing total stability. In comparison, it has been discovered that the resonance effect has a significantly bigger influence than replacement. Structures with resonance are more stable than others.
Inductive effect
Because the alkyl group has an electron-donating effect (+I), the stability of the carbocation is enhanced as the attached group’s donating ability grows.
Alkyl groups can donate more electrons inductively than hydrogen groups because they are bigger, more polarizable, and have more bonding electrons. Thus, the more alkyl substitutions there are, the more stable the carbocation.
Hyperconjugation effect
Hyperconjugation is the process of donating electrons from the parallel overlap of p orbitals with neighboring hybridized orbitals from sigma bonds. As a result, the total amount of carbon atom groups connected to the positively charged carbon determines the overall carbocation stability.
With the increase in substitution, hyperconjugation is also increased, which increases stability. The more hyperconjugation there is the more stable the system.
Electronegativity
Electronegativity is the capacity of an atom to attract or pull electrons to itself. Thus, the higher an atom’s electronegativity, the more electrons it attracts.
The stability is also directly controlled by the electronegativity of a positively charged carbon atom. When the electronegativity of the carbon atom increases, the stability of the carbocation decreases, and vice versa.
As an example, sp > sp2 > sp3. In the case of vinylic carbocation, the hybridization of the positive carbon atom is sp, with a stronger electronegativity than the sp2 alkyl carbocation hybridized carbon. As a result, the stability of the primary vinylic carbocation is lower than that of the primary alkyl carbocation.
Steric effect
Because alkyl carbocations are predominantly produced from tetrahedral alkyl halides, a link between steric relief and carbocation formation can be established. In such circumstances, the carbon-carbon bond angles change from 109028′ to 1200 during the production of carbocations. As a result, carbon with bulky groups around it is expected to benefit more from this carbocationic conversion. The stability of carbocation increases from left to right in the figure below:
Reactivity of carbocation
Reaction with nucleophile
They can combine with nucleophiles by accepting an electron pair. If all three groups on carbocation are different racemic mixture can be obtained.
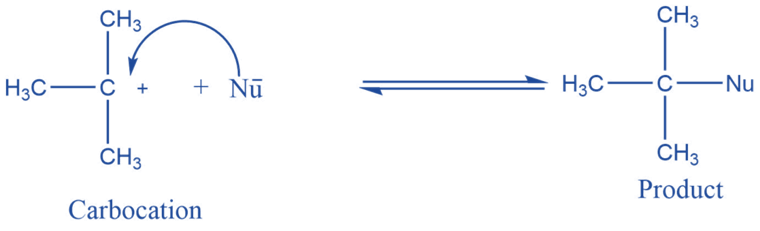
Addition reaction
They add to the double bond to create a new positively charged center.
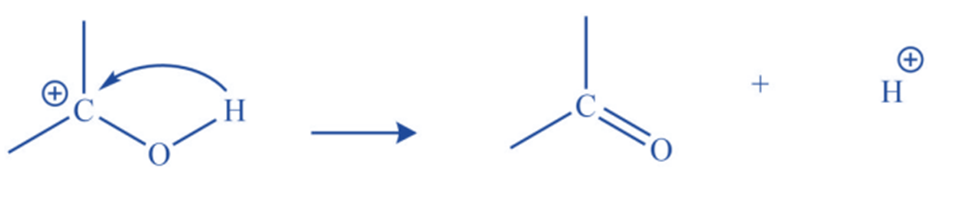
Proton removal
They may result in the removal of a proton from an adjacent carbon atom resulting in the formation of the double bond.
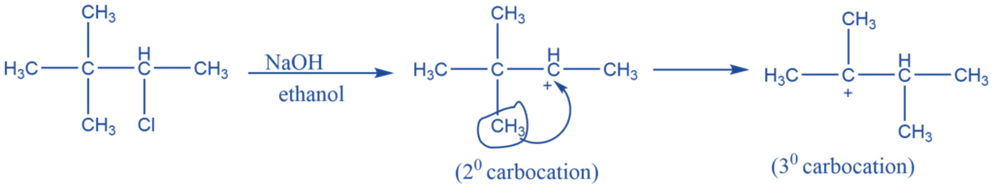
Rearrangement reaction
They undergo rearrangement to form a more stable form. In carbocation chemistry, 1-2 methyl shit or 1-2 hydride shifts are quite common to achieve a more stable counterpart. A secondary carbocation, for example, will prefer to reorganize into a more stable tertiary carbonation.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
