A vital component of contemporary chemistry is the periodic table. The periodic table was designed to provide a highly informative representation of the chemical elements. Each element is placed in the periodic table with particular parameters in mind.
For many, the Periodic Table is the symbol of chemistry. It is a single image containing all of the known elements of the universe arranged in a readable table. There are numerous patterns in the table as well. All elements appear to fit and connect to form a readable table and thus form the image of chemistry.
Definition of Periodic Table
The periodic table is an arrangement of all known elements in order of increasing atomic number and recurring chemical properties. They are organized in a tabular format, where a row represents a period, and a column represents a group. Elements are arranged in increasing atomic number order from left to right and top to bottom.
The Elements with the same valence electron configuration will have similar chemical properties.
Elements in the same period, on the other hand, will have an increasing order of valence electrons. As a result, as the atom’s energy level rises, so does the number of energy sub-levels per energy level. The first 94 elements of the periodic table occur naturally, while the remaining elements from 95 to 118 have only been synthesized in laboratories or nuclear reactors.
History of Periodic Table
It’s as common to see chemical elements arranged in the modern periodic table as it is to see a world map, but it wasn’t always that way. The art of differentiating between chemical substances underwent rapid development in the early 19th century, which led to the accumulation of a vast body of knowledge about the chemical and physical characteristics of both elements and compounds. This rapid expansion of chemical knowledge soon necessitated classification for the systematized literature of chemistry but also the laboratory disciplines by which chemistry is passed down from one generation of chemists to the next.
Contributors in the development of Periodic Table
J.W. Döbereiner demonstrated in 1817 that the combining weight, or atomic weight, of strontium, is midway between those of calcium and barium, and he later demonstrated that other such “triads” exist (chlorine, bromine, and iodine [halogens], and lithium, sodium, and potassium [alkali metals]).
Between 1827 and 1858, J.-B.-A. Dumas, L. Gmelin, E. Lenssen, Max von Pettenkofer, and J.P. Cooke expanded Döbereiner’s suggestions by demonstrating that similar relationships extended beyond the triads of elements, with fluorine added to the halogens and magnesium added to the alkaline-earth metals. At the same time, oxygen, sulfur, selenium, and tellurium were classified as one family.
Scientists such as John Newlands and Alexandre-Emile Béguyer de Chancourtois created periodic tables. These versions, however, were relatively simple, somewhat obscure, and difficult to read.
Classification of elements
J.A.R. Newlands proposed classifying the elements in increasing atomic weight order in 1864, with the elements assigned ordinal numbers from unity upward and divided into seven groups with properties closely related to the first seven elements known at the time: hydrogen, lithium, beryllium, boron, carbon, nitrogen, and oxygen.
Mendeleyev in 1869 as a result of an extensive correlation of the properties and the atomic weights of the elements, with special attention to valency (that is, the number of single bonds the element can form) stated, that the periodic law “the elements arranged according to the magnitude of atomic weights show a periodic change of properties”. Lothar Meyer had independently reached a similar conclusion, which he published after Mendeleyev’s paper was published.
Father of Periodic Table: Dmitri Mendeleev
Dmitri Mendeleev was the scientist who brought it all together (1834 to 1907). Mendeleev was a Russian-born chemist who was the first to publish a modern periodic table. His table arranged the elements according to their atomic weights (molar masses). Chemical properties were similar when the elements were ordered by their atomic weights. Mendeleev’s table was so accurate that he could predict elements that were unknown to him at the time. Germanium, gallium, and scandium were among these elements. There were some stumbling blocks on the table. Mendeleev left out important elements such as noble gases because not all of the elements had been discovered at the time of his publication
. Despite the fact that some of his predictions were incorrect, he had enough hits to establish his table as the foundation for our understanding of the elements and to confirm his place as one of the founders of modern chemistry.

Modern Periodic Law
The modern periodic law states-” The physical and chemical properties of the elements are the periodic functions of their atomic numbers”.
The number of electrons or protons in a neutral atom equals the atomic number. Scientists now had a clear understanding of quantum numbers and the electronic configuration of elements in the periodic table after learning about the fundamental unit of elements. Chemists discovered an analogy between the 94 naturally occurring chemical elements after learning about the periodic law. This analogy piqued people’s interest in the chemistry of these elements. Scientists created a variety of artificial elements. By modifying Mendeleev’s periodic table, a new periodic table based on modern periodic law was created.
The Periodic Table
The current form of the periodic table that is widely used worldwide is the long form of the periodic table. The horizontal rows in this periodic table are known as periods, and the vertical columns are known as groups.
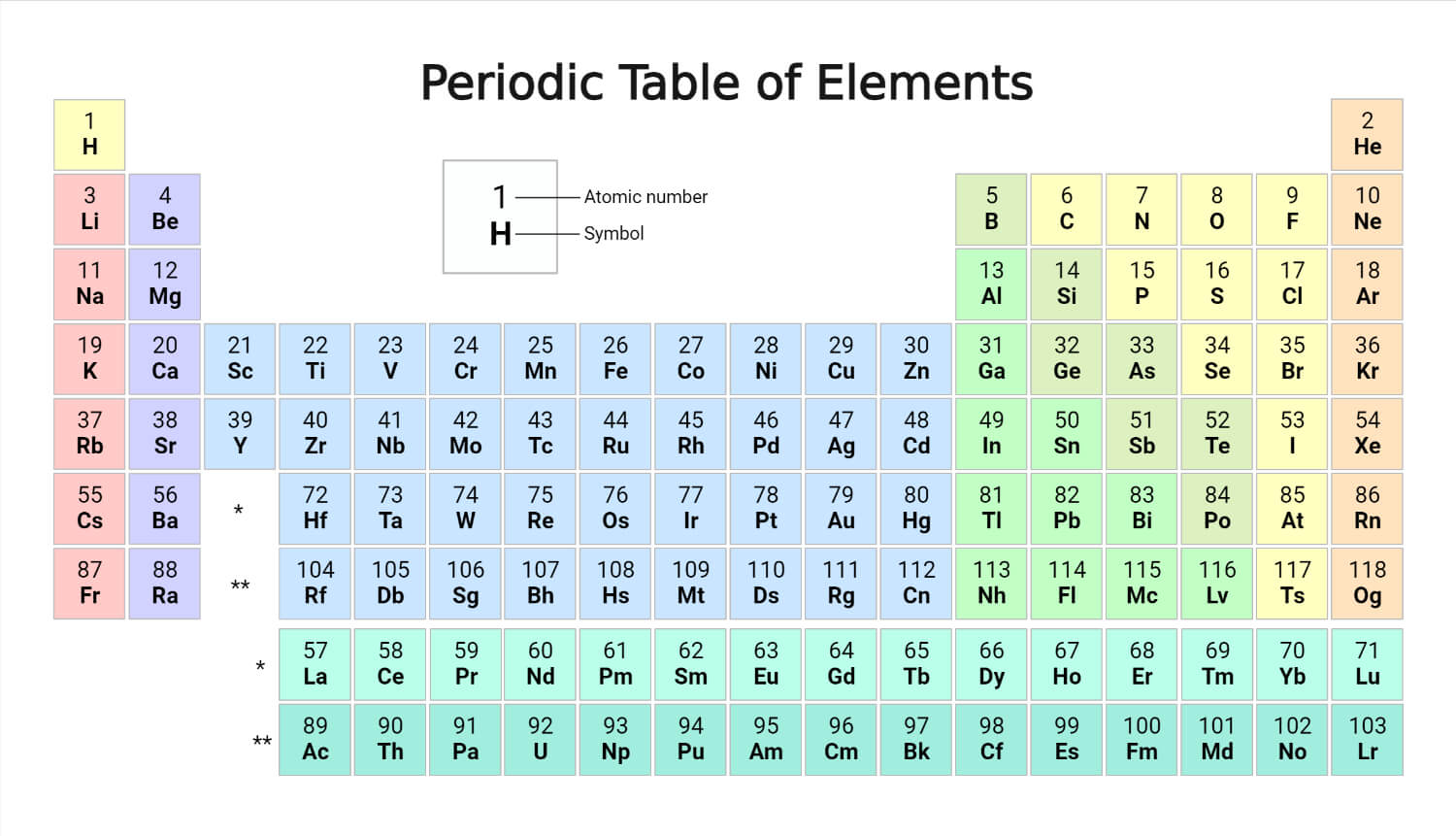
Periods
They are arranged in seven horizontal periods in the order of their atomic numbers, with the lanthanoids (lanthanum, 57, to lutetium, 71), and actinoids (actinium, 89, to lawrencium, 103) indicated separately below. The lengths of the periods vary. The hydrogen period begins with the two elements hydrogen, 1, and helium, 2. Then there are two short periods of eight elements each. There are two periods of 18 elements each.
The omission of the lanthanoids (which are indicated separately below) condenses the first very long period of 32 elements, from cesium, 55, to radon, 86, into 18 columns, allowing the remaining 18 elements, which are closely similar in properties to corresponding elements of the first and second long periods, to be placed directly below these elements. The omission of the actinoids condenses the second very long period, from francium, 87, to oganesson, 118, into 18 columns.
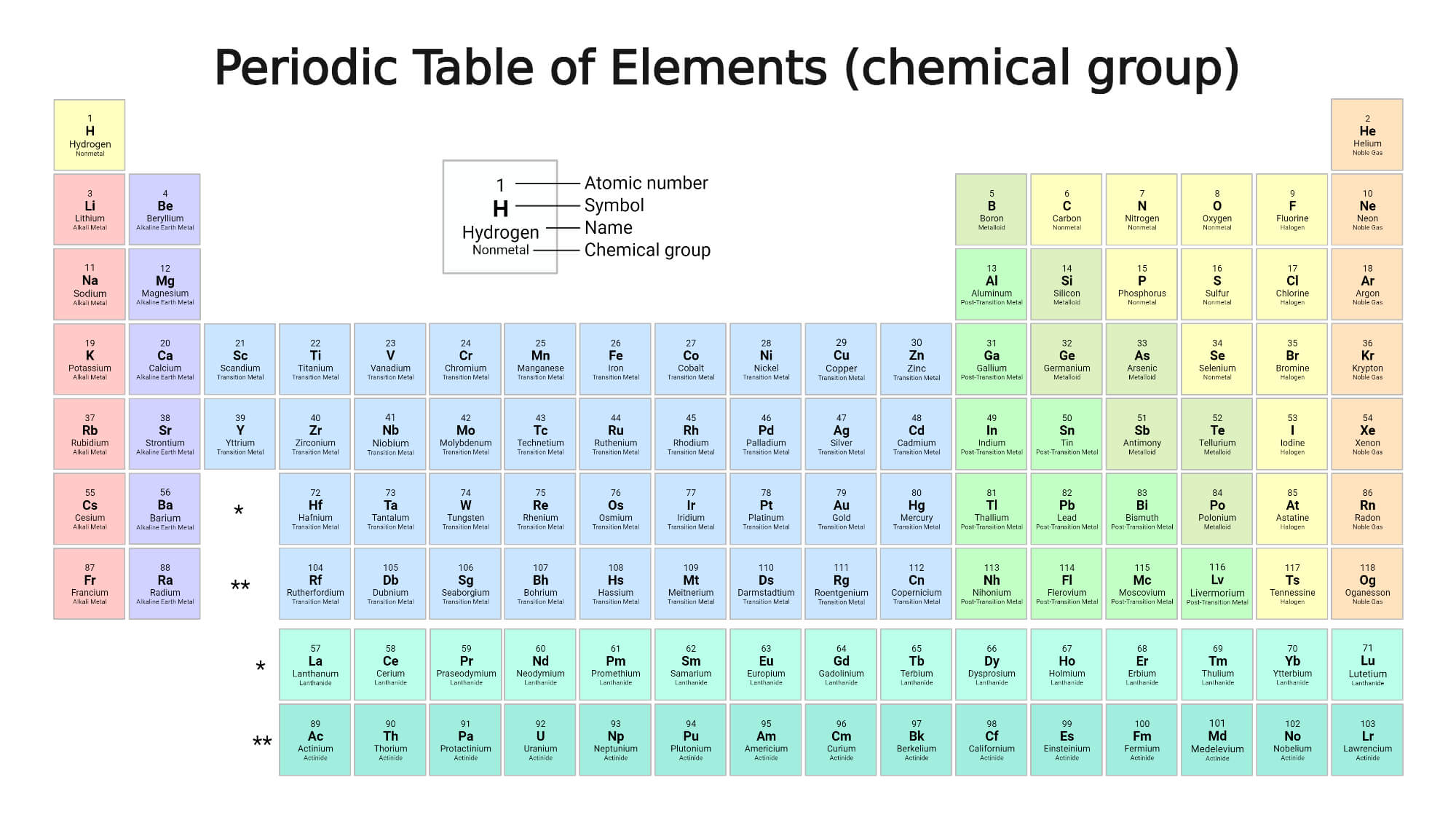
Groups
Helium, neon, argon, krypton, xenon, and radon are the six noble gases that occur at the ends of the six completed periods and make up the periodic system’s Group 18 (0) group. The seven elements lithium to fluorine and the seven elements sodium to chlorine are classified as 1 (Ia), 2 (IIa), 13 (IIIa), 14 (IVa), 15 (Va), 16 (VIa), and 17 (VIIa). The 17 elements of the fourth period, ranging from potassium (19) to bromine (35), have distinct properties and are classified as Groups 1-17 (Ia-VIIa) of the periodic system.
Periodic Table Terms
Atomic number
The number of protons in an atom’s nucleus is the element’s atomic number. The number of protons defines the element and also determines the element’s chemical behavior. As a result, the atomic number is also the number of protons, always equal to the number of electrons in the neutral atom.
Carbon atoms, for example, always have six protons, hydrogen atoms have one, and oxygen atoms always have eight. Also, an iron atom has 26 protons in its nucleus hence its atomic number is 26. Different isotopes of the same element can have different numbers of neutrons; an element can also gain or lose electrons to become charged, referred to as an ion. The atomic number may be indicated as a left subscript in the symbol representing a specific nuclear or atomic species. For example, a Hydrogen atom or nucleus (chemical symbol H) can be written as 1H.
Atomic symbol
An atomic symbol (also known as an element symbol) is an abbreviation used to represent an element (“C” for carbon, “H” for hydrogen, and “O” for oxygen, etc.). These symbols are used all over the world.
Tungsten, for example, has the symbol “W” because it is also known as wolfram. Furthermore, the atomic symbol for gold is “Au” because the Latin word for gold is “aurum.” Silver, for example, is denoted by Ag from its Latin name “Argentum.” Another example is the symbol ‘Fe,’ which represents iron and can be traced back to the Latin word for iron, “Ferrum.”
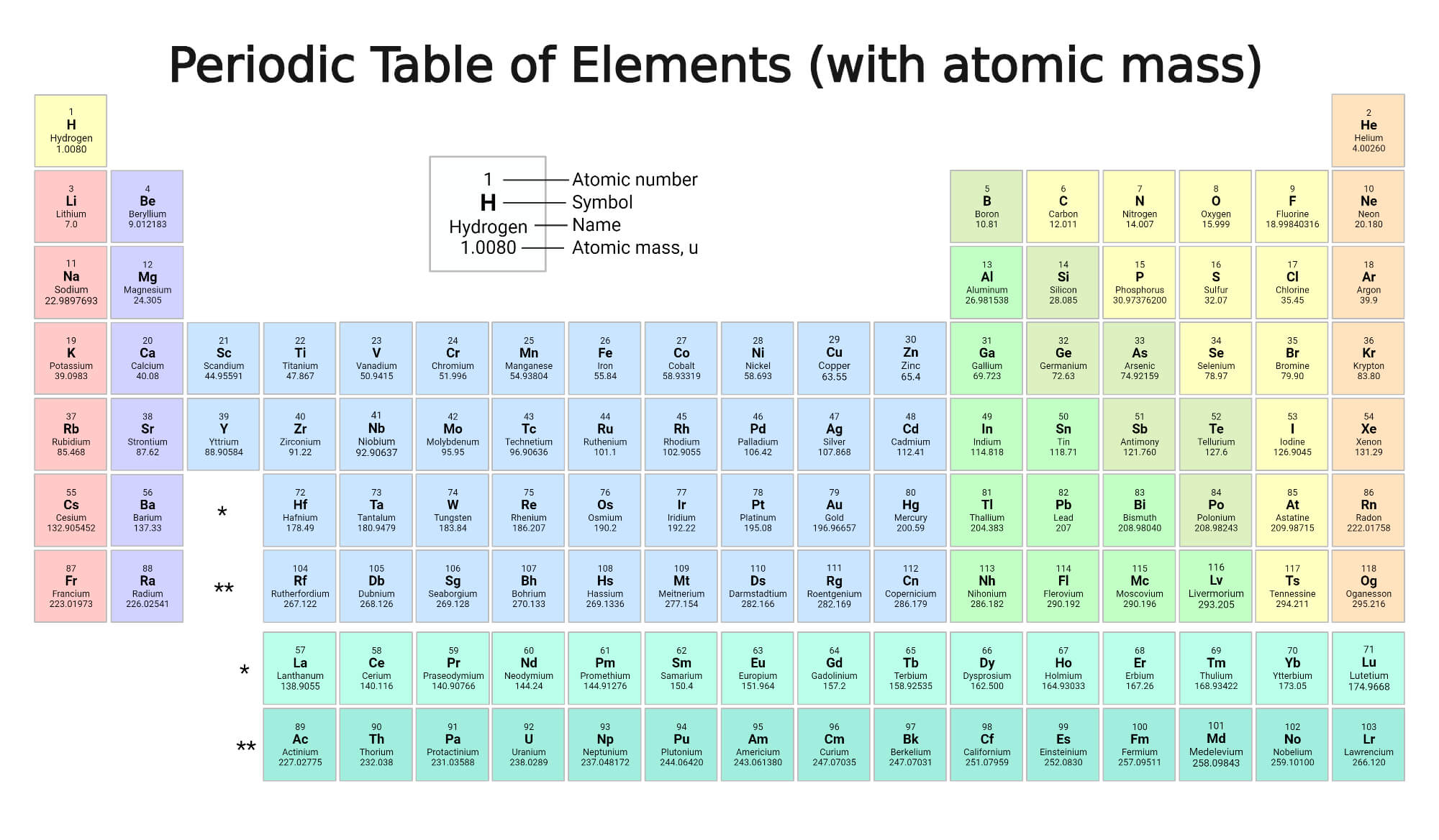
Atomic Mass
The average mass of an element written in atomic mass units is its standard atomic weight (amu). Even though each atom has roughly the same number of atomic mass units, the atomic mass on the periodic table is a decimal; this is because the number is a weighted average of the various naturally-occurring isotopes of an element based on their abundance. An isotope is an element with a different number of neutrons in its nucleus. (To determine the average number of neutrons in an element, subtract the atomic number from the atomic mass).
Since there is no “natural” abundance for lab-created trans-uranium elements, atomic mass for elements 93-118 listed on the periodic table is the longest-lived isotope.
Los Alamos National Laboratory
This non-natural category also includes superheavy elements, or those with atomic numbers greater than 104. The larger the nucleus of an atom — which grows with the number of protons inside — the more unstable that element is in general.
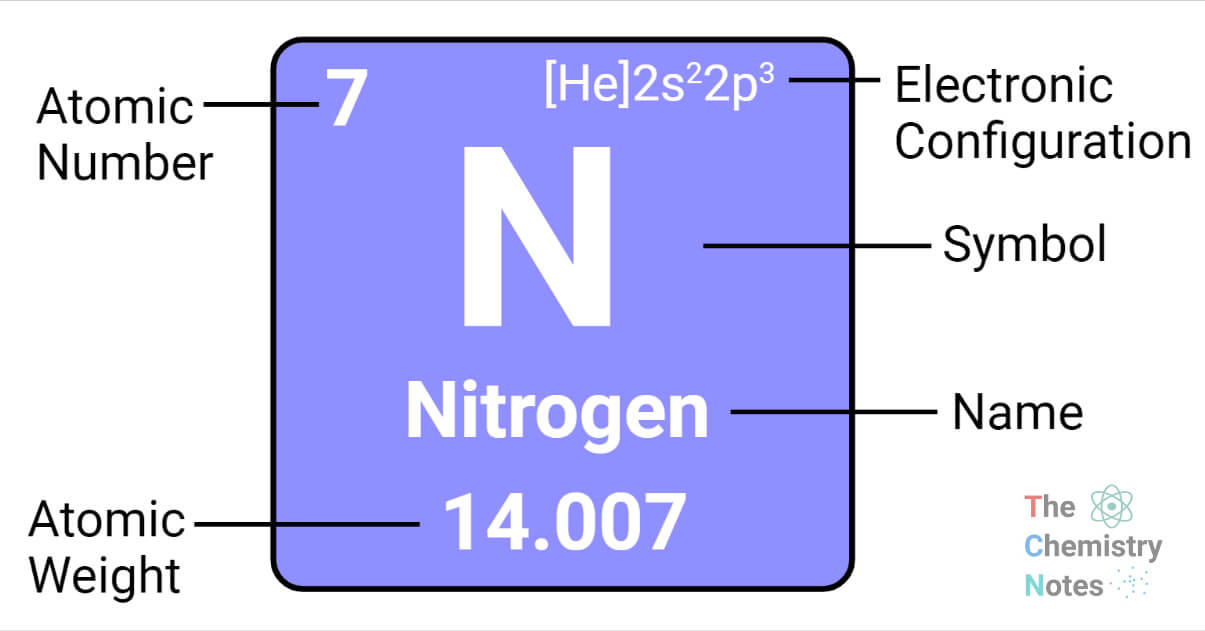
Electronic Configuration
During the development of modern atomic physics and quantum mechanics theory, a precise and detailed understanding of the electronic structure of noble gases and other atoms was obtained, fully explaining the periodic law. The long form of the periodic table, which is most commonly used, is intended to emphasize electron configurations. Because it is the outermost electrons that are primarily involved in chemical interactions between atoms, a chemist is interested in the last electron added to an atom.
In other words, the valences of the representative elements can be predicted based on the number of valence electrons they have or the number of electrons that would have to be added to achieve the same electron configuration as an atom of a noble gas.
The electron configurations of atoms in the same vertical column of the table are similar. Thus, the similarities in chemical behavior and valence observed previously for these elements correlate with similarities in their outermost electron clouds. Such similarities explain Mendeleev’s success in predicting the properties of undiscovered elements.
Importance/ Application of Periodic Table
In conclusion, the periodic table is crucial because of the way it is set up to offer a wealth of knowledge about elements and their interactions in a single, convenient reference. One of the most important tools in the history of chemistry is the periodic table.
- It concisely describes the atomic properties of every known chemical element, including the atomic number, atomic mass, and elemental relationships.
- In the periodic table, elements with similar chemical properties are arranged in columns. As an illustration, the elements in the first column, known as the alkali metals, are all metals that frequently react with a 1+ charge, react vehemently with water, and combine easily with nonmetals.
- The periodic table is an arrangement of elements in increasing mass numbers. It gives the number of neutrons and protons, as well as the average molar mass and relative mass of an element, which can be used in calculations.
- It also serves as a list of all the elements discovered to exist.
- Furthermore, it indicates whether an element is metallic or nonmetallic.
- The periodic table trends, as well as the arrangement of elements into groups and periods, assist us in identifying various trends across elements.
Limitations of Periodic Table
- Hydrogen’s placement is unclear; experts aren’t sure if it belongs in the IA group or the VIIA group. The modern periodic table has no place for isotopes of elements.
The Modern Periodic Table does not include Lanthanides and Actinides, so they are kept separately under the table.
The modern periodic table could not explain the origin of periodicity or the separation of similar elements. Some dissimilar elements have been combined. - At certain points, an element with a higher atomic mass was placed before an element with a lower mass. For example, cobalt (Co = 58.93) is usually placed before nickel (Ni = 58.71).
- Some elements within the same subgroup had different properties. For example, manganese (Mn) is combined with halogens with completely different properties.
- At certain points, an element with a higher atomic mass was placed before an element with a lower mass. For example, cobalt (Co = 58.93) is usually placed before nickel (Ni = 58.71).
- Some elements within the same subgroup had different properties. For example, manganese (Mn) is combined with halogens with completely different properties.
Elements in Periodic Table
This periodic table chart lists elements, including the element Name, Symbol, Atomic number, and Atomic mass.
| Symbol | Name | Atomic Number | Atomic Mass (amu, g/mol) |
| H | Hydrogen | 1 | 1.00797 |
| He | Helium | 2 | 4.00260 |
| Li | Lithium | 3 | 6.941 |
| Be | Beryllium | 4 | 9.01218 |
| B | Boron | 5 | 10.81 |
| C | Carbon | 6 | 12.011 |
| N | Nitrogen | 7 | 14.0067 |
| O | Oxygen | 8 | 15.9994 |
| F | Fluorine | 9 | 18.998403 |
| Ne | Neon | 10 | 20.179 |
| Na | Sodium | 11 | 22.98977 |
| Mg | Magnesium | 12 | 24.305 |
| Al | Aluminum | 13 | 26.98154 |
| Si | Silicon | 14 | 28.0855 |
| P | Phosphorus | 15 | 30.97376 |
| S | Sulfur | 16 | 32.06 |
| Cl | Chlorine | 17 | 35.453 |
| Ar | Argon | 18 | 39.948 |
| K | Potassium | 19 | 39.0983 |
| Ca | Calcium | 20 | 40.08 |
| Sc | Scandium | 21 | 44.9559 |
| Ti | Titanium | 22 | 47.90 |
| V | Vanadium | 23 | 50.9415 |
| Cr | Chromium | 24 | 51.996 |
| Mn | Manganese | 25 | 54.9380 |
| Fe | Iron | 26 | 55.847 |
| Co | Cobalt | 27 | 58.9332 |
| Ni | Nickel | 28 | 58.70 |
| Cu | Copper | 29 | 63.546 |
| Zn | Zinc | 30 | 65.38 |
| Ga | Gallium | 31 | 69.72 |
| Ge | Germanium | 32 | 72.59 |
| As | Arsenic | 33 | 74.9216 |
| Se | Selenium | 34 | 78.96 |
| Br | Bromine | 35 | 79.904 |
| Kr | Krypton | 36 | 83.80 |
| Rb | Rubidium | 37 | 85.4678 |
| Sr | Strontium | 38 | 87.62 |
| Y | Yttrium | 39 | 88.9059 |
| Zr | Zirconium | 40 | 91.22 |
| Nb | Niobium | 41 | 92.9064 |
| Mo | Molybdenum | 42 | 95.94 |
| Tc | Technetium | 43 | (98) |
| Ru | Ruthenium | 44 | 101.07 |
| Rh | Rhodium | 45 | 102.9055 |
| Pd | Palladium | 46 | 106.4 |
| Ag | Silver | 47 | 107.868 |
| Cd | Cadmium | 48 | 112.41 |
| In | Indium | 49 | 114.82 |
| Sn | Tin | 50 | 118.69 |
| Sb | Antimony | 51 | 121.75 |
| Te | Tellurium | 52 | 127.60 |
| I | Iodine | 53 | 126.9045 |
| Xe | Xenon | 54 | 131.30 |
| Cs | Cesium | 55 | 132.9054 |
| Ba | Barium | 56 | 137.33 |
| La | Lanthanum | 57 | 138.9055 |
| Ce | Cerium | 58 | 140.12 |
| Pr | Praseodymium | 59 | 140.9077 |
| Nd | Neodymium | 60 | 144.24 |
| Pm | Promethium | 61 | (145) |
| Sm | Samarium | 62 | 150.4 |
| Eu | Europium | 63 | 151.96 |
| Gd | Gadolinium | 64 | 157.25 |
| Tb | Terbium | 65 | 158.9254 |
| Dy | Dysprosium | 66 | 162.50 |
| Ho | Holmium | 67 | 164.9304 |
| Er | Erbium | 68 | 167.26 |
| Tm | Thulium | 69 | 168.9342 |
| Yb | Ytterbium | 70 | 173.04 |
| Lu | Lutetium | 71 | 174.967 |
| Hf | Hafnium | 72 | 178.49 |
| Ta | Tantalum | 73 | 180.9479 |
| W | Tungsten | 74 | 183.85 |
| Re | Rhenium | 75 | 186.207 |
| Os | Osmium | 76 | 190.2 |
| Ir | Iridium | 77 | 192.22 |
| Pt | Platinum | 78 | 195.09 |
| Au | Gold | 79 | 196.9665 |
| Hg | Mercury | 80 | 200.59 |
| Tl | Thallium | 81 | 204.37 |
| Pb | Lead | 82 | 207.2 |
| Bi | Bismuth | 83 | 208.9804 |
| Po | Polonium | 84 | (209) |
| At | Astatine | 85 | (210) |
| Rn | Radon | 86 | (222) |
| Fr | Francium | 87 | (223) |
| Ra | Radium | 88 | 226.0254 |
| Ac | Actinium | 89 | 227.0278 |
| Th | Thorium | 90 | 232.0381 |
| Pa | Protactinium | 91 | 231.0359 |
| U | Uranium | 92 | 238.029 |
| Np | Neptunium | 93 | 237.0482 |
| Pu | Plutonium | 94 | (242) |
| Am | Americium | 95 | (243) |
| Cm | Curium | 96 | (247) |
| Bk | Berkelium | 97 | (247) |
| Cf | Californium | 98 | (251) |
| Es | Einsteinium | 99 | (252) |
| Fm | Fermium | 100 | (257) |
| Md | Mendelevium | 101 | (258) |
| No | Nobelium | 102 | (250) |
| Lr | Lawrencium | 103 | (260) |
| Rf | Rutherfordium | 104 | (261) |
| Db | Dubnium | 105 | (262) |
| Sg | Seaborgium | 106 | (263) |
| Bh | Bohrium | 107 | (262) |
| Hs | Hassium | 108 | (255) |
| Mt | Meitnerium | 109 | (256) |
| Ds | Darmstadtium | 110 | (269) |
| Rg | Roentgenium | 111 | (272) |
| Cn | Copernicium | 112 | (277) |
| Nh | Nihonium | 113 | (286) |
| Fl | Flerovium | 114 | (289) |
| Mc | Moscovium | 115 | (290) |
| Lv | Livermorium | 116 | (293) |
| Ts | Tennessine | 117 | (294) |
| Og | Oganesson | 118 | (294) |
References
- https://pubchem.ncbi.nlm.nih.gov/periodic-table/
- https://chem.libretexts.org/Ancillary_Materials/Exemplars_and_Case_Studies/Exemplars/Culture/History_of_the_Periodic_Table
- https://www.britannica.com/science/periodic-table
- https://www.acs.org/content/acs/en/education/whatischemistry/periodictable.html
- https://byjus.com/periodic-table/
- https://www.lenntech.com/periodic/number/atomic-number.htm
- https://www.vedantu.com/chemistry/118-elements-and-their-symbols-and-atomic-numbers
