A carbocation is a positively charged carbon atom that is cation. Carbocations are intermediates in many organic processes. They are typically unstable and short-lived, but can be sustained by resonance or nearby functional groups that can contribute electron density. Continue reading to understand how carbocations are produced and the elements that contribute to carbocation stability.
Carbocations have a planar structure, and their trivalent carbon is known as sp2 hybridizes. The carbocation contains six electrons in its outermost electron rather than eight. The carbon atom in the carbocation is electron-poor, having only six valence electrons available for the creation of three sigma covalent bonds with the substituents.
Carbocation Stability Definition
Carbocations are highly reactive intermediates that are frequently unstable. This implies they’ll reorganize themselves quickly to build more solid structures. A carbocation stability is influenced by its structure and the presence of substituents. The tertiary carbocation is the most stable, followed by the secondary carbocation. The most unstable carbocation is its primary carbocation.
Carbocation stabilities can be determined by measuring the amount of energy required to create the carbocation by dissociating the corresponding alkyl halide, whereas the tertiary alkyl halide separates much more easily than the primary or secondary, resulting in tri-substituted carbocations that are more easily stable than di- or mono-substituted ones. Polar solvents may readily stabilize the ions, resulting in a reduced dissociation enthalpy, but there is no change in carbonation stability.
Factors Influencing Carbocation Stability
Carbocations are more stable when charged on a tertiary carbon. Carbocations, on the other hand, are the least stable when charged on a primary carbon. Carbocations’ positive charge tends to alter or shift to the most stable configuration. This is referred to as carbocation rearrangement.
Carbocation stability is influenced by three main factors. These are:
Resonance
- The carbocation stability rises as the number of resonances increases. Furthermore, the more resonating structures there are, the more stable the carbocations are.
- The delocalization of the positive charge reduces electron loss even further, increasing total stability.
- In comparison, it has been discovered that the resonance effect has a significantly bigger influence than replacement. Structures with resonance are more stable than others.
- Example of carbocation stability is cyclopropane carbocation, which is particularly stable because to the existence of moving resonance. As a result, tricyclopropane carbocation is among the most stable carbocations.
Electronegativity
Electronegativity is the capacity of an atom to attract or pull electrons to itself. Thus, the higher an atom’s electronegativity, the more electrons it attracts.
- Carbocation stability is also directly controlled by the electronegativity of a positively charged carbon atom.
- When the electronegativity of the carbon atom increases, the stability of the carbocation decreases, and vice versa.
- As an example, sp > sp2 > sp3. In the case of vinylic carbocation, the hybridization of the positive carbon atom is sp, with a stronger electronegativity than the sp2 alkyl carbocation hybridized carbon. As a result, the stability of the primary vinylic carbocation is lower than that of the primary alkyl carbocation.
Hyperconjugation and Inductive effect
- When we increase the substitution, we increase the hyperconjugation, which raises the instability. The greater the degree of hyperconjugation, the greater the stability.
- Donating electrons from the parallel overlap of p orbitals with neighboring hybridized orbitals from sigma bonds is what hyperconjugation is all about. As a result, the overall carbocation stability is determined by the total number of carbon atom groups attached to the positively charged carbon.
- The inductive effect occurs when an atom in a covalent bond draws electrons toward itself. It usually occurs with a strongly electronegative atom. Because positively charged carbon takes electrons from its substituents, it improves stability by neutralizing.
- Alkyl groups can donate more electrons inductively than hydrogen groups because they are bigger, more polarizable, and have more bonding electrons. Thus, the more alkyl substitutions there are, the more stable the carbocation.
Carbocation Stability Order
Studies have showed that 3o carbocations are more stable and need less energy to produce. As a result, 2o carbocations are more stable than 1o carbocations and need less activation energy.
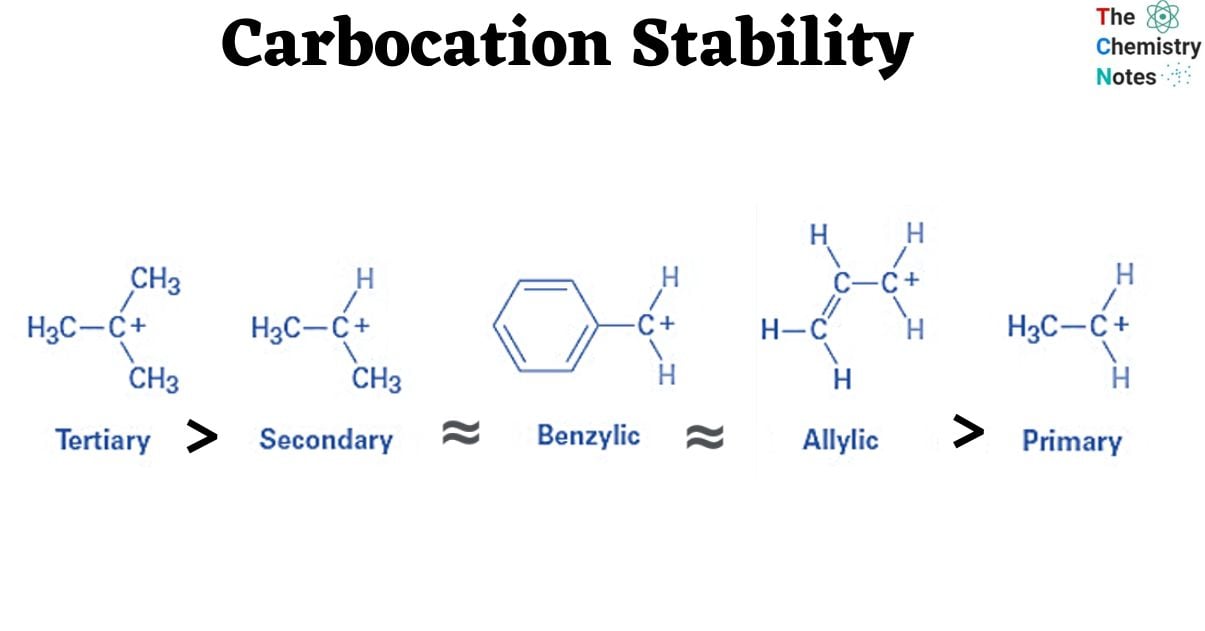
The stability of alkyl carbocations is tertiary > secondary > primary > methyl cation.
The order of stability of carbocations may also be explained by considering that alkyl groups linked to a positively charged carbon release electron density toward that carbon and thus delocalize the positive charge on the cation. Thus, the electron-releasing ability of alkyl groups attached to cationic carbon is determined by two effects: inductive effect and hyper-conjugation.
Allylic and Benzylic Carbocation Stability
Like allylic radicals, allylic carbocations have a double bond alongside the carbon that is carbon-deficient. The allyl cation is the most basic type of an allylic carbocation. Because the allyl cation has just one substituent on the positively charged carbon, it is classified as a mainly allylic carbocation. Because of the delocalization relationship of the resonance interaction between the carbon bearing the positive charge and the pie bond existing next to it, allylic carbocation is more stable than substituted alkyl carbocation.
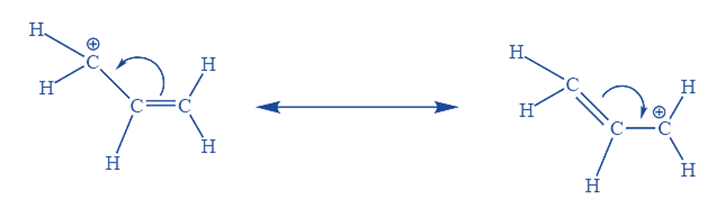
A molecule with a distributed charge is regarded more stable than a molecule with a localized charge. Experiments have also revealed that a double bond of a vinyl group present adjacently imparts stability roughly comparable to two alkyl groups. As a result, the cation 2o isopropyl cations are more stable in contrast. The positioning of the positive charge in the more essential contributing structure determines the 1o, 2o, and 3o alkyl cation.
Similarly, the electron donation in the case of benzylic carbocation is due to the resonance effect. There will be three resonance configurations possible, with the positive charge situated on one of the three aromatic carbons. The positive charge will be delocalized and not isolated around the aromatic structure. The resonance forms of benzylic carbocation are as follows:
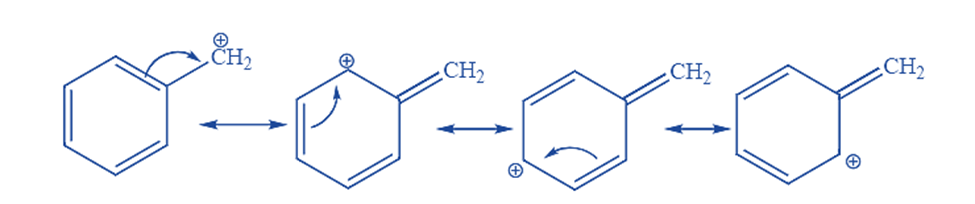
Carbocations are also stabilized by the surrounding group through hyperconjugation. A sigma bond overlapping slightly with a neighboring p orbital causes hyperconjugation. The stability of the carbocation rises with hyperconjugation. It overlaps with the carbocation’s unoccupied p orbital in this example. It can give some improved electron density to stabilize the unoccupied p orbital even without electron donation.
Frequently Asked Questions (FAQ)
Explain the stability of 3°, 2° and 1° carbocation and arrange them in decreasing order of their stability.
Comparing 3° carbocation to 2° and 1° carbocation, 3° is the most stable carbocation. The inductive effect of the alkyl groups stabilizes the 3° carbocation. Carbocations have a stability order of 3° > 2° > 1°, and 1°. The least stable is a carbocation, which is not connected to any alkyl groups.
Are allylic carbocations more stable than tertiary?
Stabilized secondary resonance carbocations are more stable than tertiary carbocations whereas stabilized primary resonance carbocations are less stable than tertiary carbocations (allyl cation, benzyl cation, and methoxymethyl cation).
What stabilizes carbocations?
Neighboring carbon-carbon multiple bonds can help to stabilize Carbocations
How does conjugation affect stability?
A conjugated structure is a system of bonded p orbitals in a molecule composed of delocalized electrons that aids in achieving stability by decreasing the overall energy of the molecule. It is generally defined as having one or more alternating bonds.
Video on Carbocation Stability
References
- https://www.chemistrylearner.com/carbocation.html
- https://www.chegg.com/learn/topic/stability-of-carbocation
- https://byjus.com/chemistry/carbocation-stability/
- https://www.vedantu.com/chemistry/carbocation-stability
- https://unacademy.com/content/jee/study-material/chemistry/carbocation-stability/
- https://chemistrytalk.org/carbocation-stability/
- https://collegedunia.com/exams/carbocation-stability-definition-resonance-stability-chemistry-articleid-705
- https://infinitylearn.com/surge/chemistry/carbocation-stability/
- https://www.aceorganicchem.com/blog/carbocation-stability/
- https://leah4sci.com/carbocation-stability-and-ranking/


Explain goc class 11
Please go through the link for content on General Organic Chemistry. https://scienceinfo.com/general-organic-chemistry-terms-topics/