Interesting Science Videos
Introduction to Bond Breaking and Bond Formation
Chemical reactions include the conversion of one or more chemicals into others. During a chemical reaction, atom bonds are broken and new bonds are established to form new molecules. This is known as bond breaking and bond formation.
Without the bonds that connect atoms, no molecule could exist in chemistry. Atoms prefer a stable complex with less energy, and since bond formation releases energy, molecules exist. Energy must be provided to the system in order to dissolve these strong connections. This value is hence referred to as bond energy.
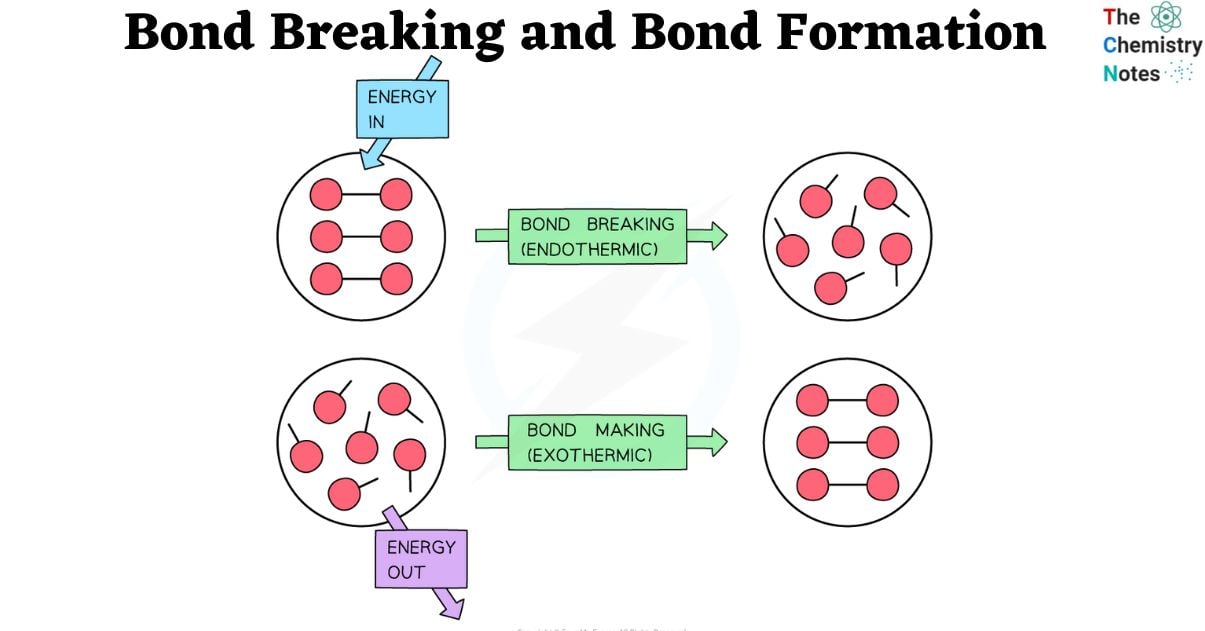
Breaking bonds is an endothermic process that constantly requires energy. However, the exothermic reaction of bond formation always results in the release of energy. In these processes, the amount of energy released or needed depends on bond energies, or the strength of a connection. Depending on the bonds of a molecule, the total reaction enthalpies might vary.
Breaking of Bond or Bond Dissociation
When energy is absorbed by a chemical system, bonds are broken. This energy can manifest as heat, light, or electricity. When energy is absorbed, the bonds between atoms weaken and finally dissolve. This is known as bond dissociation.
- The bond dissociation energy of a molecule is the amount of heat required to break bonds. The bond dissociation energy of a reaction is the energy necessary to break apart the bonds of the initial reactant. As a result, because breaking bonds takes energy, this number is always positive.
- Breaking a bond is endothermic meaning energy is required to break a chemical bond.
For instance;
ΔH - H (g)→H (g) + H (g); here ΔH=+436 kJ mol−1
One mole of covalent bonds in hydrogen molecules requires 436 kJ of energy to break.The dissociation of a bond is possible in two ways:
Homolytic Bond Breaking/Dissociation/Fission
Homolytic bond dissociation happens when the two atoms in the bond each retain one of the bond’s two electrons, resulting in two neutral atoms with unpaired electrons.
- When a particular molecule is dissociated by homolytic fission, which is also known as hemolysis, one electron is preserved by each of the original pieces of the molecule.
- Due to the fact that each chemical species maintains one electron from the bond pair, when a neutrally charged molecule undergoes homolytic fission, two free radicals are produced as the end result.
- The energy necessary to induce homolytic fission in a molecule is commonly referred to as the molecule’s homolytic bond dissociation energy.
A substantial amount of energy is often required to initiate the homolytic fission of a molecule. This is why this form of bond fission occurs only in a few instances, as stated below.
- When the molecule receives the necessary heat exposure to overcome the bond dissociation energy needed for homolytic fission.
- When carbon compounds are heated at extremely high temperatures without oxygen to help the molecule pyrolyze.
- When the molecule is exposed to UV light, which is electromagnetic energy that falls inside the ultraviolet spectrum.
Heterolytic Bond Dissociation/Breaking
During heterolytic bond dissociation, one of the bonding atoms keeps both of its electrons while the other atom transforms into a positively charged ion.
- Heterolytic fission, also known as heterolysis, is a type of bond fission in which a covalent bond between two chemical species is broken unequally, resulting in one of the chemical species retaining the bond pair of electrons (while the other species does not retain any of the bond pair’s electrons).
- When a neutrally charged molecule undergoes heterolytic fission, one of the products is positively charged, while the other is negatively charged.
- The energy required to cleave a covalent bond by heterolytic cleavage is known as the heterolytic bond dissociation energy (not to be confused with the homolytic bond dissociation energy).
- This form of cleavage is commonly observed in very unstable compounds.
Bond Formation
Bonds are formed when two or more atoms join together and share electrons to form a new bond. This is known as bond formation or bond synthesis.
The heat or energy produced when the result is created is the molecule’s bond formation energy. The energy produced during the product-forming phase of a reaction is known as the bond formation energy. Therefore, because the creation of bonds loses energy, its number is always negative.
Forming a bond is exothermic meaning energy is released when chemical bond is formed.
For instance;
H (g) + H (g) → ΔH − H (g) , here ΔH=−436 kJ mol−1
To generate one mole of covalent bonds in hydrogen molecules, 436 kJ of energy is released.There are three types of bond which are discussed here:
Ionic Bond
The attraction between positive and negative ions in a crystal forms an ionic bond, and substances bound together by ionic bonds are referred to as ionic compounds.
- Atoms having a bigger electronegativity difference create an ionic connection.
- When the ionization potentials of the two atoms differ, more ionic compounds are produced.
Because of the strong attraction between cations and anions, ionic linked molecules have the following properties:
- Ionic bonds are the most powerful of all bonds.
- Ionic bonds in the appropriate medium are the most reactive because they have charge separation.
- The melting and boiling points of the molecules with ionic bonds are high.
- Ionic bound molecules in aqueous solutions or molten state are excellent conductors of electricity. This is because ions, which function as charge carriers, are present.
Covalent Bonds
A covalent bond is created when the two involved atoms share their electrons equally. The pair of electrons participating in this type of bonding are referred to as a shared pair or bonding pair. Covalent bonds are also known as molecular bonds.
- While certain elements cannot receive electrons because of their extremely low electron affinities, others cannot because of their extraordinarily high ionization energies.
- In order for both atoms to become stable by creating an octet configuration in their respective valence shells, the atoms of these elements commonly share their electrons with those of other elements or with other atoms of the same element.
- Covalent bonds are those relationships where two distinct or similar substances exchange electron pairs.
The atoms may share many electron pairs if sharing a single electron pair does not conform to the normal valence of each element. The following traits apply to covalent bonds:
- Rarely do covalent bonds spontaneously dissolve after being created.
- Covalent bonds are directed, with the bound atoms displaying certain orientations with respect to one another.
- The majority of covalently bonded substances have relatively low melting and boiling points.
- Covalently bound substances often have lower vaporization and fusion enthalpies.
- Covalently bonded materials lack free electrons, which prevents them from conducting electricity.
Metallic Bond
A metallic bond is a sort of chemical connection established between positively charged atoms in which free electrons are shared among cations in a lattice. Covalent and ionic bonds, on the other hand, develop between two distinct atoms. The most common sort of chemical link formed between metal atoms is metallic bonding.
- Metallic bonds can be found in pure metals and alloys, as well as certain metalloids. For example, graphene (a carbon allotrope) has two-dimensional metallic bonding.
- Even pure metals may generate various kinds of chemical connections between their atoms.
Bond Energy
The bond energy is the energy consumed in breaking one mole of covalent bonds. It is the same as the energy expended in creating the same number of covalent bonds.
Bond energy is used to calculate the strength of a covalent bond. The stronger the bond to be broken, the more energy must be consumed.
- A triple bond needs the most energy to break, whereas a single bond is the simplest to break.
- According to the table, 1 mole of carbon-carbon single bonds, C-C, produces 347 kJ.
- When 1 mole of carbon-carbon double bonds, C=C, are created, 612 kJ is released.
- When one mole of carbon-carbon triple bonds, C≡C, are created, 838 kJ are released.
- Similarly, the stronger the bond is, the more energy is released.
Common examples of bond energy are given below:
| Covalent Bond | Bond energy (kJ mol−1) |
|---|---|
| H−H | 436 |
| F−F | 158 |
| Cl−Cl | 244 |
| O=O | 496 |
| C≡C | 838 |
Heat of reaction = total heat energy absorbed when old bonds are broken in the reactants – total heat energy emitted when new bonds are created in the products
When a chemical reaction occurs,
- When the total energy required to break bonds in the reactants exceeds the total energy released when new bonds are formed in the products, an endothermic reaction occurs.
- When the total energy required to break bonds in the reactants is less than the total energy released when new bonds are formed in the products, the reaction is exothermic.
An alternative way to express this is,
Er = the overall energy needed to break bonds in the reactants.Ep = overall energy released when the products establish new bonds.When Er > Ep, It is an endothermic reaction.When Er< Ep, It is an exothermic reaction.Bond Enthalpy Formula
The bond energy formula is used to compute the enthalpy of a process based on bond dissociation and formation energies. The reaction below estimates the total enthalpy by subtracting the bonds created from the ones broken.
- Find the bond enthalpy of the products (Hproducts) using a bond enthalpy table.
- Find the bond enthalpy of the reactants (H reactants) using a bond enthalpy table.
- To calculate the enthalpy of the reaction (ΔH), add the bond enthalpy of the reactants (Hreactants) and the bond enthalpy of the products (H products).
- The equation determining the bond enthalpy is: ΔH= Hreactants – Hproducts
Bond Strength Qualities and Trend in Periodic Table
The dissolution and formation energies of a bond depend on a variety of factors. A bond’s strength often hinges on:
Length of Bond
- First off, shorter bonds are often stronger and require more energy to break than longer ones since they share more valence electrons with other atoms.
- As the distance between the nuclei of the two atoms reduces, so does the bond strength.
Order of Bond
- Multiple bonds have greater dissociation and formation energy, just as triple and double bonds are both shorter than a single bond.
- Therefore, a higher bond order means that more energy is required to break the connection.
Angle of Bond
- Additionally, because molecules with bigger angles require more energy to break their bonds because shorter bond lengths come from greater angles between atoms.
- For molecules having a narrower angle between the atoms, the reverse is true.
Trend of Periodic Table
- In terms of periodic table trends relating to bond formation/dissociation energy, atoms get bigger as you travel down and left on the table.
- Because longer bonds emerge from greater atomic radii, these atoms frequently form bonds with lower dissociation and formation energies.
- For atoms with lower atomic radii/bond lengths, the periodic pattern is reversed.
Conclusion
Bond making and bond breaking are terms used to describe the simultaneous formation and breaking of bonds during chemical reactions.
The bonds between the reactants are broken during a chemical reaction, and new bonds are created to produce products. The entire bond forming and breaking process controls how much energy is changed throughout the reaction.
In order to forecast the end results of a chemical process and to create novel chemical compounds for particular uses, it is crucial to comprehend the formation and breaking of bonds.
Video on Bond Breaking and Bond Formation
References
- https://www.savemyexams.com/igcse/chemistry/cie/23/revision-notes/5-chemical-energetics/5-1-exothermic–endothermic-reactions/5-1-3-bond-breaking–bond-forming/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_I_(Cortes)/10%3A_Intro_to_Theory_of_Chemical_Reactions/10.04%3A_Bond_Breaking_and_Bond_Formation
- https://chemistrytalk.org/bond-formation-and-dissociation-energies/
- https://www.thoughtco.com/metallic-bond-definition-properties-and-examples-4117948
- https://edurev.in/question/890251/What-is-the-breaking-and-making-of-bonds-in-chemic