Chemical bonds are commonly known as intramolecular forces, which distinguishes them from intermolecular forces that occur between molecules. Intramolecular forces are the forces that hold atoms together within a molecule. Intermolecular forces are forces that exist between molecules.
What are Intramolecular Forces?
Intramolecular forces refer to the attractive forces that exist between atoms within a molecule, which serve to hold the molecule together. The three types of intramolecular interactions are covalent bonds, metallic bonds, and ionic bonds.
The three types of forces are covalent bonds, ionic bonds, and metallic bonds.
Types of Intramolecular Forces
The three primary types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds, which are also known as the types of intramolecular forces. The difference between these bond types lies in the extent of charge separation between the atoms that create the bond. The difference in electronegativity between atoms serves as a reliable indicator of charge separation.
Ionic Bond
An ionic bond is a type of chemical bond that involves the transfer of electrons between atoms. These attractive forces are known to be strong and arise from the electrostatic charges present in molecules or atoms. Ionic bonding occurs when electrons are completely transferred from one atom to another, resulting in the attraction between the resulting positive and negative ions.
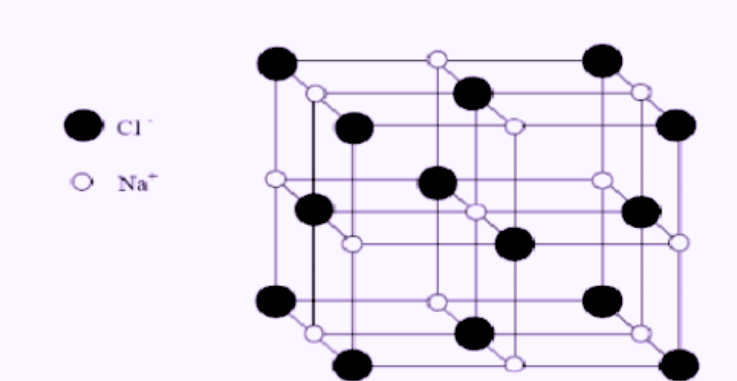
The alkali halides, such as sodium chloride (NaCl), are the most commonly occurring ionic compounds. The solid crystal of sodium chloride, commonly known as rock salt, does not consist of individual molecules of NaCl. The crystal is composed of positively charged ions (Na+) and negatively charged ions (Cl–) that are bound together by the Coulombic force, which is the attraction between oppositely charged particles.
In a cubic crystal lattice, the positive sodium ion and the negative chloride ion occupy alternate positions. Each sodium ion is surrounded by six chloride ions, and vice versa, as shown below.
Covalent Bond
A covalent bond is a type of chemical bond where two or more atoms share electrons to form a stable molecule. Electron cloud concentration between positively charged nuclei gives rise to strong attractive forces.
In other words, unstable atoms share their outermost electrons with other atoms in order to achieve stability. The diamond structure, which is composed of group IV elements like carbon, silicon, germanium, or tin, is a common crystal where atoms experience a covalent force. The carbon atoms in a diamond are arranged in a tetrahedral structure. Every carbon atom is bonded to four other carbon atoms.
Polar Covalent Bond
A polar covalent bond is a type of chemical bond that occurs when two atoms share electrons unequally, resulting in a partial positive charge on one atom and a partial negative charge on the other. This unequal sharing of electrons is due to differences in electronegativity between the two atoms involved in the bond.
A polar covalent bond is established when the electronegativity disparity between the atoms to be joined falls within the range of 0.5 and 1.9. When the electronegativity of two atoms is only slightly different, a polar covalent bond is considered to exist between them.
Non-polar Covalent Bond
A non-polar covalent bond is a type of chemical bond where two atoms share a pair of electrons equally, resulting in a balanced distribution of charge. This type of bond occurs when the electronegativity difference between the two atoms is negligible or very low.
A non-polar covalent bond is established in instances where the difference in electronegativity between the atoms involved is below 0.5. Hence, the aforementioned bond can potentially manifest between identical atoms or within atoms possessing comparable electronegativity.
Metallic Bond
Metallic crystals exhibit attractive forces known as metallic bond. For instance, in the case of sodium, which has an electronic structure of (2:8:1), when sodium atoms combine, the electrons in the outermost shell share space with the corresponding electrons in the neighboring atom to create a molecular orbital. Each sodium atom is surrounded by eight other sodium atoms, and they share electrons between the central atom and the outermost orbitals of the eight surrounding atoms. All the atoms in the lump of sodium share with each other.
Electrons have the ability to move freely within molecular orbitals, resulting in detachment of each electron from its parent atom. The electrons can be observed as a “sea of free electrons” that occupy the space between positive ions. The metal is held together by the powerful forces of attraction that exist between the positively charged ions and the surrounding sea of electrons.
How Intramolecular Forces Work?
Intramolecular forces are based on the attraction between charges that are unlike and the repulsion of charges that are like. Unlike charges refer to the negatively charged electrons and positively charged protons. Like charges refer to either separate atomic nuclei or electrons. The distribution of charge around the atomic nuclei changes when chemical bonds are formed, as compared to the distribution of charge when the atom exists independently.
Although electrons surround the nucleus of an atom, they do not completely shield it from the repulsion of neighboring nuclei and the attraction of neighboring electrons. Electrons surrounding atoms repel other electrons surrounding neighboring atoms while simultaneously being attracted to the nuclei of those atoms.
How these forces interact with each other is what ultimately determines the specific type of chemical bonds that atoms will form, as well as the resulting physical and chemical properties that molecules will possess.
Which Force is stronger? Intramolecular or Intermolecular Force
The strength of intramolecular forces is greater than that of intermolecular forces. Ionic bonds are generally considered to be the strongest intermolecular forces, although there may be some exceptions.
- The covalent bonds that exist between carbon atoms in a diamond are exceptionally strong. The strength of a bond is determined by several factors. The strength of a specific bond within a molecule can be influenced by the presence of other bonds within the same molecule.
- Molecules that possess a combination of ionic and covalent bonds generally exhibit weaker intermolecular forces or bonds compared to molecules that are purely ionic or purely covalent.
- In general, metallic bonds tend to be weaker than either ionic or covalent bonds, although there are some exceptions. Hydrogen bonding is considered to be the strongest intermolecular force.
To rank intramolecular forces from strongest to weakest, we can roughly order them as follows:
- Ionic bonds
- Polar covalent bonds
- Nonpolar covalent bonds
- Metallic bonds
- Hydrogen bonds
Potential Energy and Bonding
Chemistry exhibits a tendency towards achieving optimal stability. Greater stability of a bond is associated with a lower potential energy. It is imperative to bear in mind the fundamental rule pertaining to the strength and formation of bonds while acquiring knowledge on the subject matter.
Energy diagrams can be employed to depict physical or chemical processes owing to this association. The utilization of a graph that depicts the relationship between potential energy and interatomic distance is a valuable means of comprehending the intermolecular interactions that occur between atoms. Upon examining this graph, multiple observations can be made.
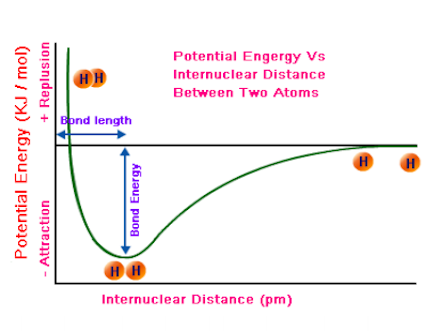
[Image source: Physics.stackexchange]
The term “equilibrium bond length” refers to the interatomic distance at which the potential energy is minimized. Let us further simplify the concept by determining the optimal distance at which the atoms exhibit maximum stability.
Bond energy refers to the quantity of energy required to dissociate two atoms that are bonded together. The disparity in potential energy between the atoms in their separated state and the atoms in their most stable phase, or equilibrium bond length, can be computed or contemplated conceptually.
The bond energy provides insight into the magnitude of the bond’s cohesive force. Typically, chemical bonds exhibiting elevated bond energies are characterized by greater strength and stability, whereas those with reduced bond energies are associated with diminished strength and stability.
The bond length refers to the spatial separation between two atoms that are bonded to each other.
Intramolecular Forces and Potential Energy
Potential Energy of Covalent Bonds
In molecular compounds featuring covalent bonds, the length of the bond is subject to influence from both the dimensions of the atoms involved and the order of the bond.
The concept of bond order pertains to the classification of chemical bonds as either single, double, or triple bonds.
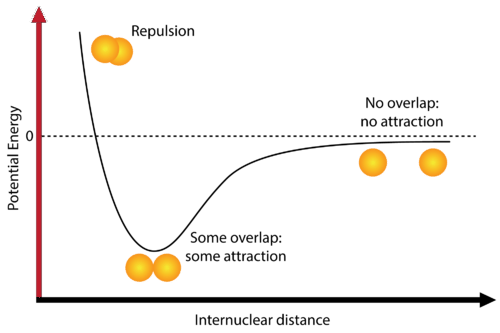
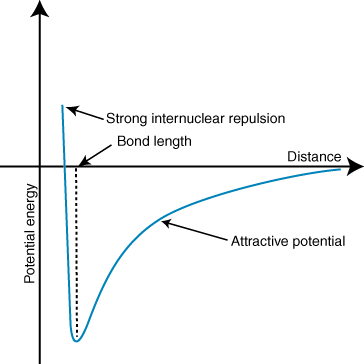
Repulsion: Electron-electron repulsion is a significant factor in the repulsion phenomenon, owing to the close proximity of atoms and the small internuclear distance. The instability of the bond results in a potential energy exceeding zero.
Attraction: The state of overlap/attraction between two atoms is considered the most stable, and the distance between the atoms at this point is commonly known as the “equilibrium bond length.” A stable bond is formed as a result of the equilibrium between the attractive and repulsive forces. It is expected that the reader has gained an understanding of the concept that potential energy is at its minimum when a bond is in a stable state.
At this juncture, the potential energy corresponds to the magnitude of energy necessary to dissociate the bond, which is commonly referred to as the bond energy.
No Attraction: The absence of bonding is attributed to the significant internuclear distance between the atoms, which precludes any form of interaction or attraction. This results in a potential energy that approaches zero.
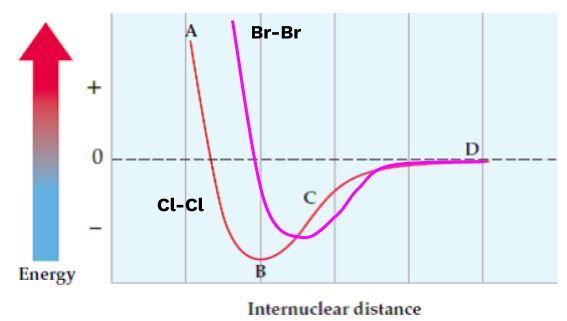
Potential Energy of Ionic Bonds
The process of forming a cation and an anion occurs when a highly electronegative atom and an electropositive atom are bonded together, resulting in the transfer of an electron from the electropositive atom to the electronegative atom. According to Coulomb’s law, the positively charged cation is drawn towards the negatively charged anion due to their opposite charges.
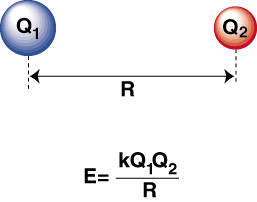
A negative energy implies the presence of an attractive interaction among the particles within the system. When the charges of two ions are of opposite polarity, they will exhibit an attractive force towards each other. In contrast, when two charges possess identical properties, they exhibit a repulsive force towards each other.
By applying this acquired knowledge, it is possible to create a graphical representation of the energy as a function of the distance between two ions with opposite charges. At significant separations, the attractive energy between the two ions is insignificant. However, as the ions approach each other, they experience an attractive force.
According to Coulomb’s law, the optimal arrangement for ions to achieve a state of minimal energy may appear to be one where they are situated near each other. Nevertheless, the data indicates that the ions experience repulsion when they are in close proximity.
In order to elucidate this observation, it is important to bear in mind that the nuclei of the ions in question possess a positive charge. As the nuclei come into proximity, they exhibit a significant repulsive force, which is responsible for the abrupt increase in potential energy observed when the ions are brought closer than the bond length.
[Image Source: Sparknotes]
The bond strength is represented by the depth of the minimum in the potential energy curve along the y-axis, while the bond length is indicated by the distance at the energy minimum along the x-axis. By applying Coulomb’s law and considering the bond length, it is possible to make reasonably accurate predictions regarding the potency of an ionic bond.
Upon conducting a sequence of aforementioned computations, it can be inferred that ionic compounds resulting from ions with higher charges exhibit greater bond strength. Additionally, it can be deduced that ionic compounds featuring shorter bond lengths tend to form stronger bonds.
Examples of Intramolecular Forces
Table Salt (Sodium Chloride)
Sodium chloride, commonly known as table salt, is a prime illustration of intramolecular interactions. The formation of a stable sodium chloride structure is attributed to the ionic bond that binds the atoms of sodium and chlorine together. Upon donating an electron, sodium undergoes a process of ionization and acquires a positive charge. Chlorine undergoes reduction by accepting an electron, resulting in the acquisition of a negative charge.
The electrostatic attraction between the negatively charged anion, specifically chlorine, and the positively charged cation, specifically sodium, results in the chemical compound known as sodium chloride or table salt.
Toothpaste
Sodium fluoride is a compound characterized by an ionic bond between the metallic element sodium and the non-metallic element fluoride. Sodium undergoes oxidation by relinquishing an electron, resulting in the acquisition of a positive charge, whereas fluoride undergoes reduction by accepting the donated electron, leading to the acquisition of a negative charge.
The phenomenon of electron transfer results in the establishment of a polarity gradient, which in turn generates an attractive force responsible for the cohesive bonding of the compound.
Diamond
The formation of diamond is attributed to the application of high temperature and pressure on coal for an extended duration. Essentially, it can be described as a vast covalent bond comprised of carbon atoms. The element carbon possesses a valency of four electrons, indicating that it has the capacity to either relinquish or acquire four electrons in order to achieve an octet configuration. Rather than retaining the electrons, it has a tendency to distribute them among neighboring atoms.
Consequently, each carbon atom forms covalent bonds with four adjacent carbon atoms. The transfer of electrons among carbon atoms exemplifies the presence of intramolecular forces in the natural world.
Lye
Lye is an alkaline solution that finds its application in the production of cleansing agents such as soaps and other cleaning products. Sodium hydroxide is the chemical nomenclature for lye. The formation of sodium hydroxide is attributed to the presence of an ionic bond between a metallic element and a chemical compound. The sodium ion is characterized by a positive charge of +1, while the hydroxide atom bears a negative charge of -1. The establishment of a strong bond between two entities and the subsequent formation of a new product with distinct characteristics is attributed to the equal and opposite nature of their charges.
Alloys
Metal alloys are produced through the establishment of a metallic bond among the atoms of the constituent elements, whereby the freely moving electrons facilitate the bonding process. The metallic bonding, which exists between the two or more distinct elements being used to form an alloy, increases the conductivity and strength of the newly formed material. Alloying is a metallurgical technique that involves the deliberate introduction of specific impurities into a pure metal with the aim of modifying and improving its properties.
Frequently Asked Questions (FAQ)
What are the factors affecting Potential energy?
The potential energy of a molecule is determined by two key factors, namely the internuclear distance and the attractive or repulsive forces. The potential energy is commonly plotted as a function of the internuclear distance. As the distance between the atoms varies, the attraction or repulsion between them also changes, thereby influencing the potential energy.
What are the examples of intramolecular forces?
Intramolecular forces, which are responsible for holding atoms together within a molecule, include covalent bonds, ionic bonds, and metallic bonds. An instance of an intramolecular force can be observed in the ionic bond formed between sodium and chlorine in salt. The intermolecular forces that exist between two hydrogen molecules can be classified as intramolecular forces, which are inherent to the covalent bond.
What is the basic difference between intermolecular and intramolecular forces?
Intramolecular forces refer to the cohesive forces that bind atoms together within a molecule. Intermolecular forces are the attractive or repulsive forces that occur between molecules.
Video on Intramolecular Force
References
- Bader, R. F. W.; Henneker, W. H. (1965). “The Ionic Bond.” Journal of the American Chemical Society. 87 (14): 3063–3068. doi:10.1021/ja01092a008
- https://www.sparknotes.com/chemistry/bonding/ionic/section1/
- IUPAC (2019). “Intramolecular.” Compendium of Chemical Terminology (the “Gold Book”) (2nd ed.). Oxford: Blackwell Scientific Publications. ISBN 0-9678550-9-8. doi:10.1351/goldbook
- https://www.simply.science/images/content/chemistry/states_of_matter/force_of_attraction/conceptmap/intra_molecular_forces.html
- https://sciencenotes.org/intramolecular-forces/
- https://www.chemistrylearner.com/chemical-bonds/intramolecular-forces
- King, Matcha (1976). “Theory of the Chemical Bond”. JACS. 98 (12): 3415–3420. doi:10.1021/ja00428a004
- Oxtoby, David W.; Gills, H. P.; Campion, Alan (2012). Principles of Modern Chemistry (7th ed.). Belmont, Calif.: Brooks/Cole Cengage Learning. ISBN 978-0-8400-4931-5.
- https://www.iitianacademy.com/ap-chemical-unit-2-2-intramolecular-force-and-potential-energy/
- Zumdahl, Steven S.; Zumdahl, Susan A. (2007). Chemistry (7th ed.). Boston: Houghton Mifflin. ISBN 978-0618713707.
- https://www.studocu.com/row/document/kabale-university/solid-state-physics/2intermolecular-forces-and-potential-energy/13965080
- https://library.fiveable.me/ap-chem/unit-2/structure-ionic-solids/study-guide/3khaTI6A3tdnaMTMJy2u
- https://studiousguy.com/intramolecular-forces-types-examples/
- https://www.iitianacademy.com/ap-chemical-unit-2-2-intramolecular-force-and-potential-energy/

