Covalent Bond Definition
The covalent bond is a type of chemical bond between the atoms of the same or different elements by the mutual sharing of pairs of electrons.
- Covalent bonding is the result of attractive forces between the electrons and the nuclei as well as the repulsive forces between the shared electrons.
- In a covalent bond, two atoms usually share the number of electrons which is termed as the covalency of the atoms.
- Like in all forms of bonding, atoms involved in covalent bonding share electrons in order to achieve a stable electronic configuration.
- Even though covalent bonding is mostly formed of electrostatic forces, other interactions like -bonding, -bonding, bent binds, three-center two-electron bonds, and three-center four-electron bonds are also involved.
- Covalent bonds only form when the total energy of the formed compounds is lesser than that of the separated atoms.
- The concept of covalent bonding was put forth by the American chemist G. N. Lewis in 1916.
- Covalent bonding is far more common than other types of bonding, and some degree of covalency can be observed in compounds with other types of bonding as well.
- Unlike other types of bonding, covalent bonding can exist between two atoms of the same elements resulting in the molecular form of the element.
- A specific type of covalent bond called coordinate covalent can also be observed in some interactions where the shared pair of electrons between two atoms come from a single atom.
- Covalent bonding between carbon atoms forms the basis of organic chemistry as this property enables the formation of thousands of organic compounds found in living bodies.
- In organic compounds, the electrons might be shared with more than two atoms, in which case the electrons are said to be localized in order to achieve a stable structure.
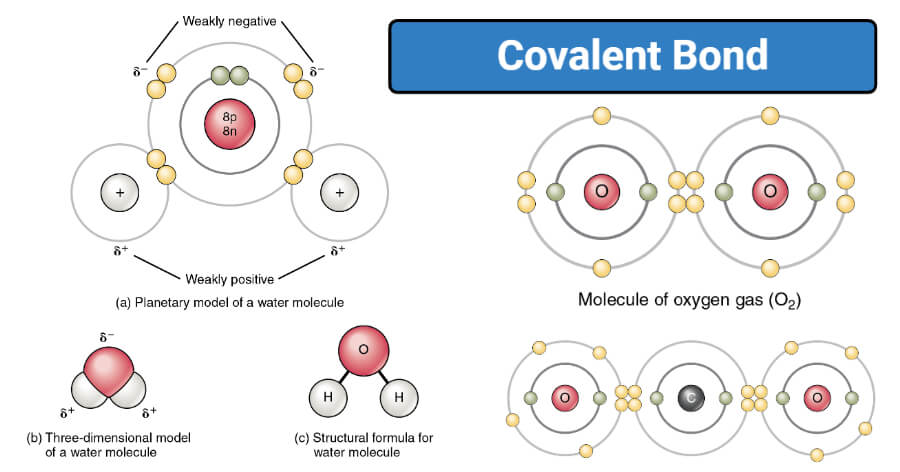
Image Source: Openstax.
Condition for Covalent Bonding
The following are the conditions for the formation of covalent bonding between chemical substances;
- The most important condition for the formation of a covalent bond is that the energy of the resulting compound should be less than the separated atoms.
- The two atoms involved in the formation of the covalent bond should be electronegative, and true covalent bonds are formed between atoms with similar electronegativities.
- The atoms should have high ionization energy so that they do not easily lose electrons from their outermost orbit.
- The elements involved in the formation of the covalent bond should have more than 4 electrons in their outermost orbit so that a stable configuration can be obtained by sharing particular pairs of electrons.
Covalent bond properties
Covalent bonds and covalent compounds have the following properties;
1. State
- Covalent compounds can exist in all three states of matter depending on the type and number of covalent bonds present between atoms.
- Covalent compounds that exist in the gaseous state are mostly bonded together by weak electrostatic forces, whereas those occurring in solid-state have stronger forces.
- Some common examples of covalent compounds in different states include H2O in a liquid state, CO2 in a gaseous state, and diamond in solid-state.
2. Boiling and melting point
- The boiling and melting point of covalent compounds is less than ionic compounds as these depend on the degree of attraction between the atoms or the strength of the bond.
3. Electrical conductivity
- Covalent compounds cannot conduct electricity under normal conditions except graphite which is a good conductor of electricity.
- As covalent compound does not form ions in an aqueous or molten state, these compounds cannot conduct electricity.
4. Directional nature
- Covalent bonds are directional in nature due to the overlapping of certain orbitals that are oriented towards a particular axis.
- The overlapping of the orbitals determine the strength of the bond and thus, effective overlapping result in stronger bonds.
- The direction of the bond formed depends on the direction of the orbitals involved in the bond formation.
- The s-orbitals are symmetrical and thus, can form covalent bonds in any direction, but the p-orbitals are directed at a particular axis and form stable bonds in that particular direction.
- Different covalent compounds exhibit isomerism due to the directional nature of the bonds.
5. Solubility
- Covalent compounds do not dissolve in polar solvents but can dissolve in non-polar solvents.
- Some covalent compounds might dissolve in water due to the formation of intermolecular hydrogen bonding with water molecules.
- The insolubility of covalent compounds in polar solvents is due to the lack of polarity of the bond.
Types of covalent bonds
Covalent bonds are of different types depending on the number of covalent bonds present in the compound. The number of covalent bonds further depends on the number of electrons shared.
1. Single Covalent Bond
- Single covalent bonds are formed by the sharing of a single pair of electrons by the participating atoms. A single covalent bond is indicated by (-).
- Compounds with single covalent bonds have lesser density, and the bonds are weaker by the bonds are more stable than other types of covalent bonds.
- The bond length is the longest in single covalent bonds as two electrons hold the nucleus with a lesser force.
- Single covalent bonds occur in elements that are farther away from each other in the periodic table.
- An example of a single covalent bond is the covalent bond between hydrogen and chlorine atoms in HCl.
2. Double Covalent Bond
- Double covalent bonds are formed by the sharing of two pairs of electrons between the participating atoms. A double covalent bond is indicated by (=)
- The strength of a double covalent bond is in between the single and triple covalent bonds. Similarly, the bond length is smaller than the single covalent bond but greater than the triple covalent bond.
- A double covalent bond occurs in elements that are closer in the periodic table with four or more electrons in their outermost orbit.
- The double bonds are usually less stable than single bonds because the shared electrons have higher reactivity.
- An example of double covalent bonds is the bonding between carbon and oxygen atoms in CO2.
3. Triple Covalent Bond
- A triple covalent bond is formed by the sharing of three pairs of electrons between the participating atoms. A triple covalent bond is indicated by (≡).
- Triple covalent bonds are the strongest covalent bonds as the nuclei are held together by three pairs of electrons. The bond length is the shortest as the bonds are pulled closer by stronger forces.
- The triple covalent bond is the least stable bond as the increased electron density result in higher reactivity of the bond.
- This type of bond occurs between the atoms of the same element or elements that are very close in the periodic table.
- An example of a triple covalent bond is the bonding between two nitrogen atoms in the N2 molecule.
How are covalent bonds formed?
- Covalent bonds are formed in compounds where none of the atoms involved is metal atoms with sufficiently low ionization energy to make the loss of electrons possible.
- As a result, the atoms involved in the compounds tend to share electrons so that both the atoms can attain a stable electronic configuration.
- Covalency is more prevalent in elements lying towards the right of the periodic table (mostly nonmetals).
- The concept and formation of a covalent bond can be explained in terms of Lewis terms. In Lewis’s terms, a covalent bond is a shared pair of electrons.
- The most important condition for the formation of the covalent bond is the lowering of the energy of the atoms involved.
- The decrease in energy occurs when the electrons are shared as the electrons lie between two attracting nuclei resulting in lower energy than when attracted by a single nucleus.
- As a result of the sharing of electrons, both the atoms attain a stable noble gas configuration with lower energy.
- The shared pair of electrons in a covalent bond is termed as bonding pair whereas the remaining electrons on the outermost orbit (if present) are termed as lone pairs.
- The bonding pairs of electrons (one or more depending on the number of bonds) are attracted by two atomic nuclei of the participating atoms so that they can remain shared among the atoms.
- A covalent bond usually forms when the electronegativities of two atoms are too small for the transfer of electrons to occur.
- In covalent compounds, the bonding electrons are the glue holding the atoms of the molecules together.
What are Polar covalent bonds?
- The polar covalent bond is a type of covalent bond where the shared pair of electrons are unequally distributed among the two atoms.
- Polar covalent bonding results in a slight electrical dipole moment as one of the atoms becomes slightly positively charged, and the other becomes slightly negatively charged.
- The charges developed on the atoms are less than a complete unit charge which is denoted by delta plus (δ+) and delta minus (δ-).
- The molecules formed by polar covalent bonds can even interact with other molecules with a dipole moment.
- Polar covalent bonds are considered a mix between the covalent bond and ionic bond. Most of the covalent bonds are polar bonds when the atoms involved have some difference in their electronegativities.
- An example of a polar covalent bond is the covalent bond between hydrogen and oxygen in a water molecule.
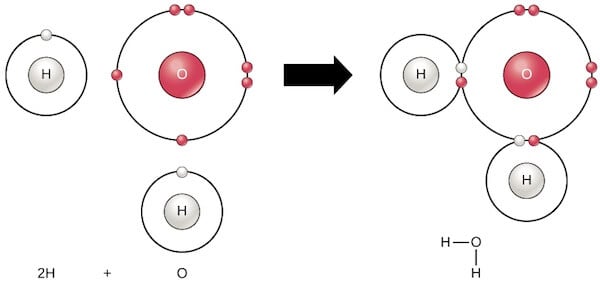
Figure: Polar covalent bonding in Water (H2O). Image Source: Khan Academy.
What are Non-polar covalent bonds?
- The non-polar covalent bond is a type of covalent bond where the shared pair of electrons are equally distributed between the two participating atoms.
- In a non-polar covalent bond, the number of electrons shared by the two atoms is the same.
- Non-polar covalent bonds are usually formed between two identical non-metal atoms or different atoms with negligible electronegativity.
- In some cases, non-polar covalent bonding can form from the polar covalent bonds with the bonding electrons arranged in a particular manner.
- Non-polar covalent bonding is the ideal covalent bonding resulting in compounds that can be distinguished from polar compounds in different properties.
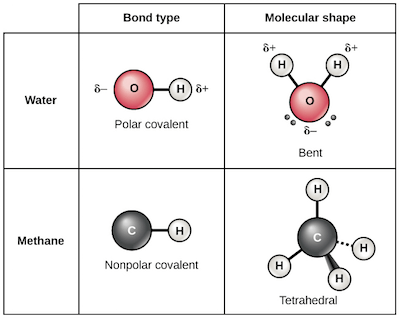
Figure: Polar and non-polar covalent bonds examples. Image Source: Khan Academy.
What are the Coordinate covalent bonds?
- A coordinate covalent is a chemical linkage between two atoms where a lone pair of electrons are donated by a donor atom and accepted by an acceptor atom. This type of bond is also called a dative bond.
- The coordinate covalent bond is a type of covalent bond as the bond is formed by the sharing of electrons, but the electrons are shared by a single atom.
- The symbol (→) is used to denote a coordinate covalent bond directed at the acceptor atom.
- The coordinate covalent bond is a two-center two-electron type covalent bond that is commonly formed between the metal ions and ligands.
- An example of a coordinate covalent bond is the bonding between the nitrogen atom and hydrogen atom in NH4+.
Examples of Covalent Bonds
1. Covalent bonding in HCl
- In HCl, a covalent bond is formed between a hydrogen atom and a chlorine atom. The covalent bond in HCl is a polar covalent bond as the electronegativities of the two atoms are different.
- Even though hydrogen and chlorine are farther away from each other in the periodic table, the difference in electronegativities is too small to be ionic.
- One element is shared by each of the atoms involved so that hydrogen and chlorine can achieve a stable electronic configuration.
- As the covalent bond is polar, the hydrogen and chlorine atoms develop a slight positive and negative charge, respectively.
2. Covalent bonding in O2
- Covalent bonding can also occur between the atoms of the same element to produce the molecular form.
- A double covalent bond exists between the two oxygen atoms where each atom shares a pair of electrons.
- The covalent bond in O2 is a nonpolar covalent bond as there is no difference in electronegativity between the two atoms and thus, allows mutual sharing of electrons.
- The oxygen molecules formed exists in a gaseous state as the non-polar covalent bond is weaker than the polar covalent bond.
Applications of Covalent Bonds
- There are different covalent compounds found on the plant that are used for different purposes. Compounds ranging from gases like O2, N2, and CO2 to liquid acids like HCl and H2SO4 are all covalent compounds formed from covalent bonds.
- One of the most critical applications of covalent bonding is the formation of thousands of organic compounds that are essential for the structure and function of living beings.
- Linkages like glycosidic linkages between sugar molecules and peptide linkages between amino acids are covalent linkages that enable the formation of macromolecules.
- Hydrogen bonding is also a type of covalent bonding that enables different properties of the bond that are exploited for different purposes.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- Robert J. Ouellette, J. David Rawn. Structure of Organic Compounds. Principles of Organic Chemistry, Elsevier. 2015. Pages 1-32. https://doi.org/10.1016/B978-0-12-802444-7.00001-X.
- Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 2.1, Covalent Bonds. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21595/
- 1% – https://www.technologyuk.net/science/matter/chemical-bonding.shtml
- 1% – https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/covalent-bond
- 1% – https://www.quora.com/Why-is-H2O-a-covalent-bond
- 1% – https://study.com/academy/lesson/polar-and-nonpolar-covalent-bonds-definitions-and-examples.html
- 1% – https://gradeup.co/jee-main-short-notes-chemical-bonding-i-80e43510-e861-11e6-b04c-1c9be0d6cce8
- 1% – https://byjus.com/jee/covalent-bond/
- <1% – https://www2.estrellamountain.edu/faculty/farabee/BIOBK/BioBookCHEM1.html
- <1% – https://www.yourdictionary.com/covalent-bond
- <1% – https://www.thoughtco.com/definition-of-dative-bond-604985
- <1% – https://www.sciencedirect.com/topics/physics-and-astronomy/inorganic-compounds
- <1% – https://www.sciencedirect.com/topics/chemistry/covalent-bond
- <1% – https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/covalent-bond
- <1% – https://www.quora.com/What-are-the-conditions-for-the-formation-of-an-ionic-bond
- <1% – https://www.preservearticles.com/chemistry/polar-and-non-polar-covalent-bonds/444
- <1% – https://www.lifepersona.com/what-is-a-polar-covalent-bond-with-examples
- <1% – https://www.differencebetween.com/difference-between-coordinate-covalent-bond-and-vs-covalent-bond/
- <1% – https://www.chemguide.co.uk/atoms/bonding/doublebonds.html
- <1% – https://www.britannica.com/science/covalent-bond
- <1% – https://www.britannica.com/science/chemical-bonding/Covalent-bonds
- <1% – https://www.bbc.co.uk/bitesize/guides/zyt6w6f/revision/4
- <1% – https://www.bbc.co.uk/bitesize/guides/zcvy6yc/revision/1
- <1% – https://www.answers.com/Q/How_are_coordinate_covalent_bonds_different_from_other_covalent_bonds
- <1% – https://web.chem.ucsb.edu/~devries/chem1C/handouts/zumdahl_chemprin_6e_csm_ch14.pdf
- <1% – https://quizlet.com/65133751/chemistry-7-1-7-2-flash-cards/
- <1% – https://quizlet.com/3904586/single-double-and-ttriple-covalent-bonds-flash-cards/
- <1% – https://quizlet.com/269165651/chemistry-chapter-8-covalent-bonds-flash-cards/
- <1% – https://quizlet.com/185010252/chemistry-chapter-6-review-flash-cards/
- <1% – https://quizlet.com/174638542/honors-chemistry-unit-6-covalent-bonds-flash-cards/
- <1% – https://quizlet.com/160275474/ch-4-polar-non-polar-covalent-bonds-flash-cards/
- <1% – https://chem.libretexts.org/Courses/Windward_Community_College/BIOC_141%3A_Fundamentals_of_Biochemistry_(Colmenares_and_Ashburn)/04%3A_Covalent_Bonding_and_Simple_Molecular_Compounds/4.1%3A_Covalent_Bonds
- <1% – https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/Coordinate_(Dative_Covalent)_Bonding
- <1% – https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Fundamentals/Ionic_and_Covalent_Bonds
- <1% – https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Worksheets/Worksheets%3A_Inorganic_Chemistry/Structure_and_Reactivity_in_Organic_Biological_and_Inorganic_Chemistry/01%3A_Introduction_to_Atoms/1.6%3A__The_Periodic_Table_and_Periodic_Trends
- <1% – https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/2%3A_The_Chemical_Foundation_of_Life/2.1%3A_Atoms%2C_Isotopes%2C_Ions%2C_and_Molecules/2.1I%3A_Covalent_Bonds_and_Other_Bonds_and_Interactions
- <1% – https://answers.yahoo.com/question/index?qid=20090805122018AAXmurX
- <1% – https://answers.yahoo.com/question/index?qid=20090520042830AASWSQX
- <1% – http://hyperphysics.phy-astr.gsu.edu/hbase/Chemical/bond.html
