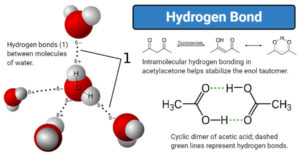
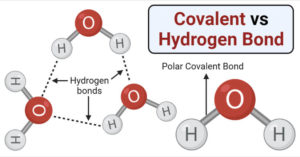
Intermolecular Forces in Liquid: 3 Important Types
Forces of attraction and repulsion between molecules are known as intermolecular forces. The interactions between molecules of a substance are mediated by these forces. Intermolecular forces are responsible for most … Read more