Hydrogen Bond Definition
A hydrogen bond is an attractive force between the hydrogen atom of one molecule bound and more electronegative atoms of the same molecule or other molecules.
- The hydrogen bond is weaker than a covalent bond, and thus, the bond length is longer. The hydrogen bond is denoted by a dotted line (…).
- Electronegative atoms generally involved in hydrogen bonding are fluorine, nitrogen, and oxygen. Hydrogen bonds with sulfur, chlorine, and carbon are also observed, but these bonds tend to be weaker.
- The hydrogen bond is a type of polar covalent bond where the pair of electrons is unequally distributed between hydrogen and another atom resulting in small charges on both atoms.
- It has been debated that the bond has some ionic contributions due to the formation of partially charged ions.
- Even though hydrogen bond is an electrostatic dipole-dipole interaction, the bonding is achieved in order to achieve a stable electronic configuration in both atoms.
- Hydrogen bonding is an essential interaction as it provides different properties to the compounds formed. Besides, as a hydrogen bond can exist between two molecules, it is involved in the structure and function of the compounds formed.
- It is considered a weaker bond than an ionic bond and covalent bond, but it is stronger than Van der Waal’s force.
- Hydrogen bonding exists in both organic and inorganic compounds, but the hydrogen bonding in organic compounds occurs only when the carbon atoms are bound to a more electronegative atom.
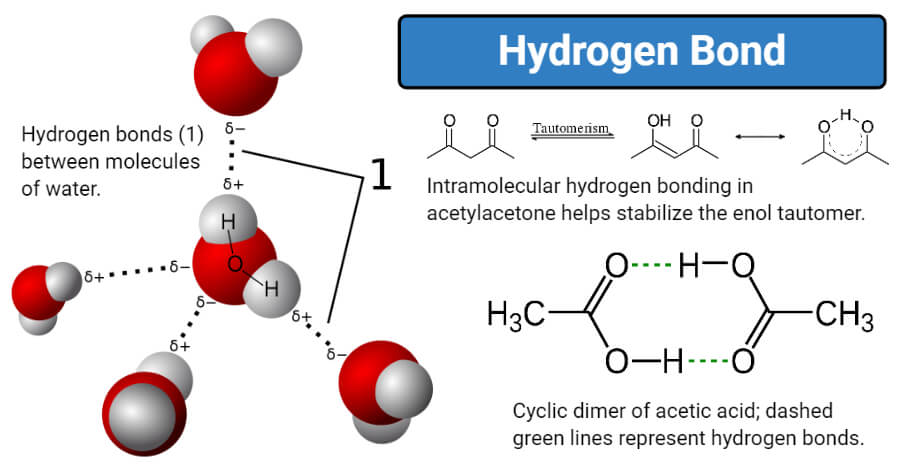
Image Source: Wikipedia.
Condition for Hydrogen Bonding
The following are the conditions for the formation of hydrogen bonding between chemical substances;
- Out of the two atoms, one should be a hydrogen atom as it is highly electropositive and small in size, which increases the polarity of the bond.
- The other atoms bound to hydrogen should be highly electronegative in order to generate a dipole moment.
- The size of the electronegative atoms should be small as atoms with smaller size have a greater electrostatic attraction.
- In the case of intermolecular hydrogen bonding, the atom of the other molecule should be electronegative and bound to a hydrogen atom.
Hydrogen bond properties
Hydrogen bonding results in a variation in the physical properties of the compounds. Some of which are:
1. Boiling and melting point
- Hydrogen bonds affect the boiling and melting point of compounds as the greater hydrogen bond results in increased intermolecular attraction.
- As a result, a large amount of heat energy is required to break down the forces and result in melting or boiling.
- In a compound, intermolecular hydrogen bonding causes molecules to exist in associated molecules.
- The association increases the size and molecular mass of the compound resulting in increased Van der Waal’s force.
- Thus, compounds having more hydrogen bonds have a higher boiling and melting point.
2. Density and surface tension
- Hydrogen bonding also affects other physical parameters like density, surface tension, and even viscosity.
- The compounds have greater associations or linkages between molecules, which results in increased density of the compounds.
- The surface tension also increased as the molecules on the surface are bound to one another via these bonds.
3. Physical State
- Compounds with hydrogen bonds exist either in the liquid or gaseous state. Compounds with stronger bonds exist in the liquid state whereas those with weaker hydrogen bonds exist in the gaseous state.
- The strength of the bond depends on the electronegativity of the atom bound to hydrogen. In H2O, hydrogen bonds are stronger due to the higher electronegativity of oxygen whereas those in H2S are weaker as sulfur is less electronegative than oxygen.
4. Molecular association
- Compounds with hydrogen bonds form aggregates as a result of a weak association between molecules.
- The formation of dimers and trimers in different compounds occurs due to hydrogen bonding.
5. Solubility
- Hydrogen bonding is responsible for the solubility of different compounds as it is involved in the hydration of anions in aqueous solutions.
- The hydration contributes to the solvent property of the compounds and helps dissolve different ionic compounds.
Types of hydrogen bonds
Depending on the occurrence of the hydrogen bonding, hydrogen bonds are of two types;
1. Intramolecular hydrogen bonds
- The intramolecular hydrogen bond is the hydrogen bond formed between the hydrogen atoms and electronegative atoms like nitrogen, oxygen, and sulfur within the same molecule.
- For the formation of an intramolecular hydrogen bond, hydrogen and an electronegative atom should be present in molecules within close proximity.
- In some molecules like ethylene glycol, even though the atoms are present far apart, the molecule geometry of the compounds enables them to indulge in hydrogen bonding.
- Intramolecular hydrogen bonding in a molecule has a pronounced effect on the molecular structure and properties of the compounds.
- The bonding doesn’t, however, have a pronounced effect on the physical properties of the compound overall.
2. Intermolecular hydrogen bonds
- Intermolecular hydrogen bonding is a type of hydrogen bonding that exists between two distinct molecules either of the same compound or different compounds.
- This type of bonding can occur between any number of same or different molecules as long as hydrogen, and electronegative atoms are present.
- Like intramolecular hydrogen bonding, intermolecular hydrogen bonding also occurs when the two molecules are in close proximity to one another.
- Intermolecular hydrogen results in different properties of the compounds like increased surface tension and viscosity. These changes are often drastic.
How do hydrogen bonds form?
- Hydrogen bonds are formed by the interaction between a hydrogen atom and electronegative atoms like nitrogen, oxygen, and fluorine.
- In the bond, hydrogen and the electronegative atom, both share an electron with each other to achieve a stable electronic configuration.
- In the bond, the shared pair of electrons is displaced towards the more electronegative atoms due to higher electron affinity.
- The unequal distribution of electrons between the atoms results in the generation of charge in the atoms.
- The electronegative atom develops a negative charge as it has a comparatively high electron density whereas the hydrogen atom develops a positive charge due to the low electron density.
- The charge developed on the atoms are smaller than a complete unit charge and thus are termed as delta positive (δ+) and delta minus (δ-).
- As the atoms become polar, they have a dipole moment which allows them to interact with each other as well as other similar atoms.
- The electrostatic force or dipole-dipole interaction between the charged atoms results in the formation of the hydrogen bond.
- In the case of intermolecular hydrogen bonding, the slightly positively charged hydrogen atom can interact with a slightly negatively charged electronegative atom of a different molecule.
- This type of interaction acts as a linkage for the binding of different molecules of either the same compounds or two different compounds.
- The strength of the hydrogen bond depends on the degree of charge developed on the atoms, which, in turn, depends on the extent of displacement of the shared electrons.
- Thus, the strength of the hydrogen bond within a molecule or between molecules depends on the electronegativity of the atom involved in the bond formation.
Hydrogen bonding in water
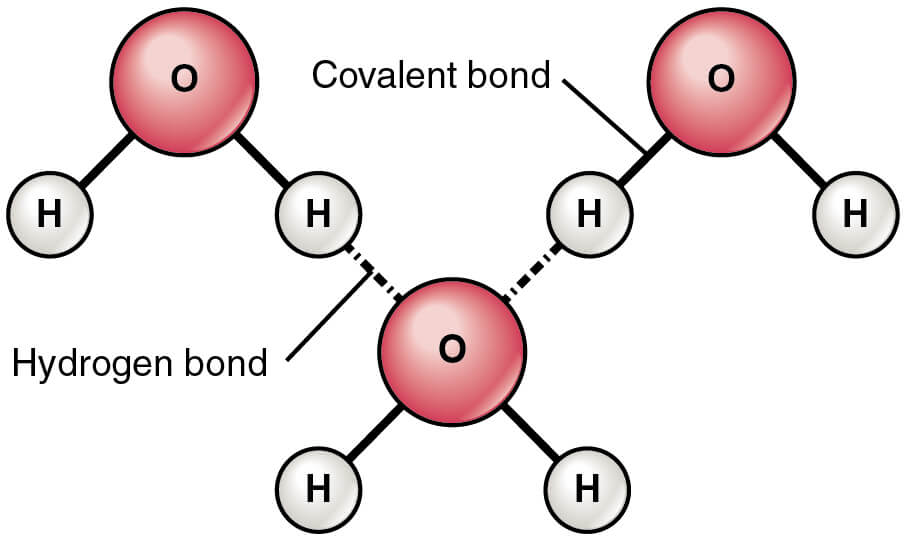
Figure: Hydrogen Bonds between Water Molecules. Image Source: Openstax.
- In water, the hydrogen bonds are the attractive forces that keep the molecular mass of the compound together.
- Hydrogen bonding exists in both liquid and solid ice state where the molecules are held together by both intermolecular and intramolecular interactions.
- Within the water molecule, two hydrogen bonds are present, each of which has the dipole moment of 1.5D.
- The hydrogen bonding in water is responsible for the different physical properties of water. The boiling point and melting point of water is higher than other similar hydrogen compounds due to the formation of intermolecular hydrogen bonding.
- Water can exist in the solid-state in the form of crystals as the water freezes due to the hydrogen bonding.
- Because of the extensive hydrogen bonding present between water molecules, it can exist in the liquid state over a wide range of temperatures.
- The universal solvent property of water is also due to hydrogen bonding. The atoms in water can readily form hydrogen bonding with electronegative atoms in different solutes, allowing their solubility.
- Hydrogen bonding within water also enables the hydration of anions in an aqueous solution, which adds to the ability of water to act as a good solvent of ionic compounds.
Hydrogen bonding in DNA
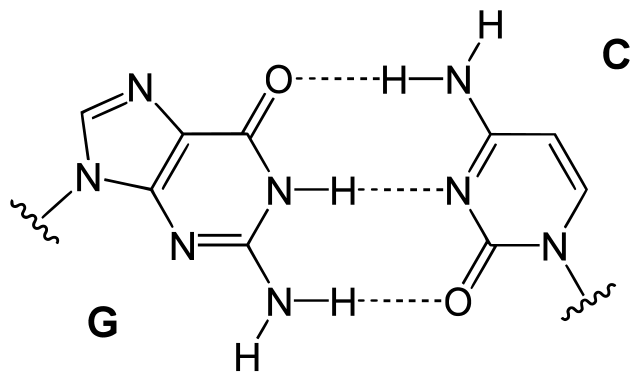
Figure: Hydrogen bonding in DNA. Hydrogen bonding between guanine and cytosine, one of two types of base pairs in DNA. Image Source: Yikrazuul.
- Hydrogen bonding is essential in the structure and function of different nucleic acids, including DNA.
- The hydrogen bond is imperative and responsible for the reduplication, transcription, and translation of DNA.
- Nucleotide bases in DNA are linked by hydrogen bonds in the base pairs like adenine in one strand and thymine in the other. These bonds keep complementary nucleotides together in the form of a helix.
- Each of the four bases can also form hydrogen bonds with molecules in the external environment.
- Even though the internal and external hydrogen bonds in DNA are weak, a large number of bonds in the molecule makes it stable.
- The hydrogen bonds between bases also place the bases in the interior of the double helix.
- The number of hydrogen bonding in different base pairing is different as cytosine and guanine are held together by three hydrogen bonds, but adenine and thymine are held together by two hydrogen bonds.
- Hydrogen bonding also stabilizes the DNA double helix across the helix axis but not in the direction of the axis.
- The deoxyribose-phosphate backbone acts as a hydrogen bond acceptor through phosphate and sugar atoms. This hydrogen bond help stabilizes the DNA double helix across the axis.
Examples of Hydrogen Bonds
1. Hydrogen bonding in proteins
- Hydrogen bonds are present in the secondary structure of proteins that hold the oxygens of the backbone with amide hydrogens.
- The α-helix secondary structure of proteins is formed as a result of the spacing of amino acid residues regularly between positions i and i+4.
- The beta-sheet structure is formed by the presence of hydrogen bonds alternating residues on each participating strand.
- Some hydrogen bonds also result in the tertiary structure of proteins through the interaction of alkyl groups.
- In multimeric proteins, hydrogen bonds play an essential role in the stability between the subunits.
- Hydrogen bonding is also involved in protein folding, influencing the quaternary structure of some proteins.
2. Hydrogen bonding in NH3
- Ammonia molecules have three hydrogen bonds within a molecule which is supported by the electronegativity of nitrogen and its small size.
- But the number of hydrogen bonding in ammonia is limited as the electron contains a single lone pair of electrons.
- The hydrogen bonding in water is stronger than that of ammonia as the electronegativity of oxygen is higher than nitrogen.
- Each hydrogen bond in ammonia has a dipole moment of 1.5D which influences the structure of the molecule.
- Molecules might also exist in intermolecular hydrogen bonding with other ammonia molecules as well as molecules of other compounds.
Applications of Hydrogen Bonds
- Different organic and inorganic compounds containing hydrogen bonds have different applications in different areas.
- Hydrogen bonding is essential for the structure and function of nucleic acids like DNA and RNA. In both RNA and DNA, the base pairing is stabilized by different numbers of hydrogen bonds.
- Different structures of proteins are stabilized by hydrogen bonds either within the protein molecule or with external molecules.
- Hydrogen bonding in water is responsible for different properties of water like high boiling point, surface tension, and solvent nature.
- Hydrogen bonds have an essential role in drug discovery as most oral drugs have about 8-10 hydrogen bonds.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors, Pvt. Ltd.
- Jeffrey G.A., Saenger W. (1994) The Role of Hydrogen Bonding in the Structure and Function of the Nucleic Acids. In: Hydrogen Bonding in Biological Structures. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-85135-3_20
- 1% – https://www.sciencedirect.com/topics/physics-and-astronomy/hydrogen-bonds
- 1% – https://www.britannica.com/science/chemical-bonding/The-hydrogen-bond
- 1% – https://quizlet.com/99507662/chapter-4-homework-flash-cards/
- 1% – https://qsstudy.com/chemistry/effects-hydrogen-bond-properties-compounds
- 1% – https://en.wikibooks.org/wiki/Structural_Biochemistry/Chemical_Bonding/Hydrogen_bonds
- 1% – https://en.glosbe.com/en/en/intramolecular%20hydrogen%20bond
- 1% – https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_(Miessler_Fischer_Tarr)/03%3A_Simple_Bonding_Theory/3.04%3A_Hydrogen_Bonding
- 1% – https://byjus.com/jee/hydrogen-bonding/
- <1% – https://www.youtube.com/watch?v=1vm3od_UmFg
- <1% – https://www.thoughtco.com/what-causes-hydrogen-bonding-603991
- <1% – https://www.thoughtco.com/difference-between-organic-and-inorganic-603912
- <1% – https://www.thoughtco.com/definition-of-hydrogen-bond-605872
- <1% – https://www.slideshare.net/RamyaAS2/protein-94237386
- <1% – https://www.sciencedirect.com/topics/chemistry/solubility
- <1% – https://www.quora.com/What-is-the-difference-between-hydrogen-bonds-and-polar-bonds
- <1% – https://www.priyamstudycentre.com/2020/03/hydrogen-bonding.html
- <1% – https://www.idc-online.com/technical_references/pdfs/chemical_engineering/Intermolecular_Forces_Boiling_and_Melting_Points.pdf
- <1% – https://www.differencebetween.com/difference-between-intermolecular-and-vs-intramolecular-hydrogen-bonding/
- <1% – https://www.chemistrylearner.com/chemical-bonds/hydrogen-bond
- <1% – https://www.britannica.com/science/water/Structures-of-ice
- <1% – https://www.brainkart.com/article/Importance–Strength–Types-of-Hydrogen-bonds—Intermolecular-Forces_2808/
- <1% – https://www.answers.com/Q/Does_ammonia_have_a_dipole_moment
- <1% – https://quizlet.com/cw/458050312/303-covalent-bonding-flash-cards/
- <1% – https://quizlet.com/49277390/biology-1-exam-1-review-flash-cards/
- <1% – https://quizlet.com/120876954/properties-of-water-due-to-hydrogen-bonding-flash-cards/
- <1% – https://quizlet.com/114038063/chapter-9-bonding-flash-cards/
- <1% – https://pediaa.com/how-do-polar-and-nonpolar-molecules-interact-with-each-other/
- <1% – https://en.wikipedia.org/wiki/Hydrogen-bonding
- <1% – https://en.wikipedia.org/wiki/Alpha_helix
- <1% – https://byjus.com/biology/difference-between-dna-and-rna/
- <1% – https://askinglot.com/which-has-a-higher-boiling-point-h2o-or-h2s
- <1% – http://chemistry.elmhurst.edu/vchembook/161Ahydrogenbond.html
