Covalent Bond Definition
A covalent bond is a type of linkage between the atoms of the same or different elements as a result of the mutual sharing of electrons.
- The bond is formed as a result of the electrostatic force of attraction between the nuclei and the electrons.
- Covalent bonds are formed by the sharing of electrons so that the atoms involved can attain a stable electron configuration.
- The covalency of an atom is defined by the number of electrons shared between the atoms in a covalent bond.
- Even though covalent is considered a simple chemical bonding, it might involve other forms of bonding like -bonding, -bonding, and multiple center bonding.
- Chemical bonding results in a release of energy which decreases the total energy of the atoms involved. The reduction in energy generates a more stable structure and supports the formation of the bond.
- The number of covalent bonding between two atoms can single, double or triple bonds depending on the electronic configuration of the atoms and the shared pair of electrons.
- A single covalent bond consists of two electrons shared between the atoms, whereas a double bond consists of four electrons.
- Covalent bonds are mostly formed between nonmetals and metalloids, where the donation of electrons is not possible.
- Covalent bonds are usually strong, with bond strength ranging between 100 to 1100 kJ/mol.
- The bond strength depends on the covalency of the atoms, which is greatest among the atoms with similar electronegativities.
- Covalent bonds, however, are not as strong as ionic interactions, and covalent bonds might exhibit some ionic character.
- Covalent bonds are indicated by solid lines connecting the atoms sharing the electrons. The number of lines represents the number of covalent bonds.
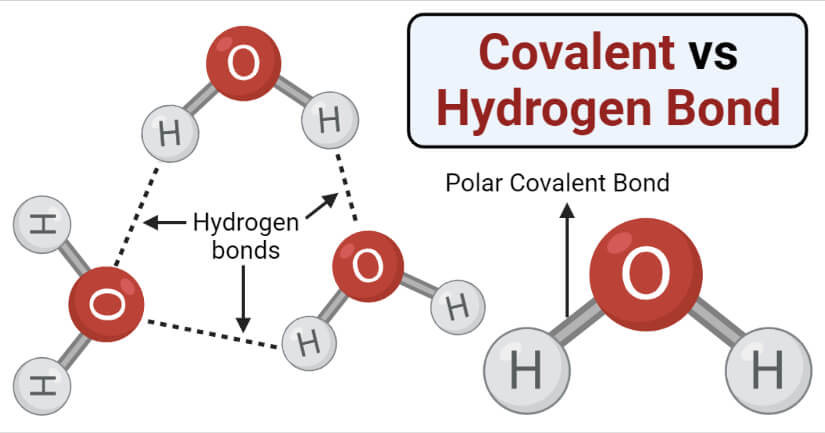
Hydrogen Bond Definition
A hydrogen bond is a type of chemical bonding between a hydrogen atom of one molecule and a more electronegative atom of the same or different molecule.
- The electronegative atom involved in hydrogen bond includes fluorine, oxygen, sulfur, chlorine, and nitrogen. The hydrogen bond between hydrogen and sulfur, chlorine, and carbon are usually weaker due to lesser electronegativities.
- A hydrogen bond is a polar covalent bond where the shared pair of electrons are unequally distributed between the two atoms. Hydrogen bond results in small charges on the atom.
- Hydrogen bonding is an electrostatic dipole-dipole interaction, however, the interaction can result in the formation of a stable electronic configuration in the atoms.
- Hydrogen bonding between atoms is responsible for various properties in compounds that would not be possible otherwise.
- These bonds occur in both organic and inorganic compounds, but the hydrogen atom must be bonded to a more electronegative atom.
- For the formation of the hydrogen bond, the electronegative atoms should be small as atoms with smaller sizes usually have a higher electron affinity.
- Hydrogen bonding is of two types depending on the molecules involved; intramolecular hydrogen bonding is the hydrogen bond between the hydrogen atom and electronegative atoms within the same molecule.
- Intramolecular hydrogen bonding is the hydrogen bond between the hydrogen atom and electronegative atom of two different molecules of the same or different compound.
- Hydrogen bonds are formed due to the difference in electronegativity between the atoms, which results in charges smaller than a complete unit charge.
- The charges on the atom give rise to a dipole moment which allows them to interact with each other and generate an electrostatic attraction.
11 Major Differences (Covalent vs Hydrogen Bond)
| Characteristics | Covalent Bond | Hydrogen Bond |
| Definition | A covalent bond is a type of linkage between the atoms of the same or different elements as a result of the mutual sharing of electrons. | A hydrogen bond is a type of chemical bonding between the hydrogen atom of one molecule and a more electronegative atom of the same or different molecule. |
| Strength | Covalent bonds are stronger than hydrogen bonds. | Hydrogen bonds are weaker than covalent bonds. |
| Bond strength may vary between 100 to 1100 kJ/mol. | Bond strength may vary between 5 to 50 kJ/mol. | |
| Nature | Covalent bonds are intermolecular interactions. | Hydrogen bonds can be either intermolecular or intramolecular. |
| Atoms | Covalent bonds are formed between nonmetals and metalloids. | Hydrogen bonds are formed between hydrogen atoms and other electronegative atoms. |
| Polarity | Covalent bonds can be polar or nonpolar. | Hydrogen bonds are polar bonds. |
| Breakdown | Covalent bonds are comparatively harder to break down. | Hydrogen bonds are comparatively harder to break down. |
| Bond length | Covalent bonds have a shorter bond length. | Hydrogen bonds have a longer bond length. |
| Represented by | Covalent bonds are represented by solid lines. | Hydrogen bonds are represented by dotted lines (…). |
| Types | Covalent bonds are of two types; polar and nonpolar covalent bonds. | Hydrogen bonds are of two types; intermolecular and intramolecular hydrogen bonding. |
| Examples | Covalent bonding can be found in molecules like O2, CH4, etc. | Hydrogen bonding can be found in molecules like H2O, NH3, etc. |
Examples of Covalent Bond
Covalent bonding in CH4
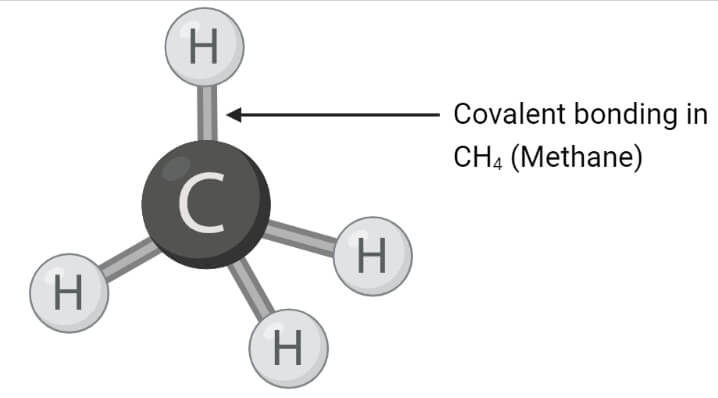
- Methane molecule consists of a single carbon atom and four hydrogen atoms. The carbon and hydrogen atoms are linked together by covalent bonds.
- There are four single covalent bonds between carbon and hydrogen atoms, resulting in a tetrahedral shape with a bond angle of 109°.
- Since carbon atoms have four valence electrons, they attain a stable electronic configuration by forming four covalent bonds with hydrogen atoms.
- The strength of the carbon-hydrogen covalent bond is strongest in methane molecules among all hydrocarbons.
- The carbon-hydrogen bond is nonpolar due to the minimal difference in electronegativities between carbon and hydrogen.
- All the covalent bonds in methane are identical, and the electronic structure of the molecule is described by the molecular orbitals resulting from the overlapping of the valence orbitals in carbon and hydrogen atoms.
Examples of Hydrogen Bond
Hydrogen bonding in DNA
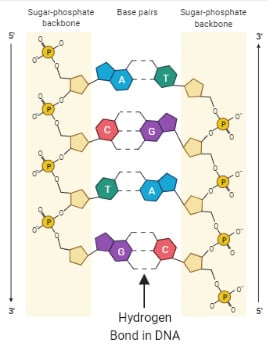
- Hydrogen bonds are present in nucleic acids like DNA and RNA, where the nucleotide bases are linked to each other via such bonds.
- The hydrogen bonds are responsible for keeping the complementary bases linked to one another to form the helical structure of the DNA.
- The nucleotide bases can also form hydrogen bases with other molecules in the environment like water.
- An individual hydrogen bond might be weak to hold the nucleotide bases together, but there are two or three hydrogen bonds between the nucleotides which make the structure more stable.
- The deoxyribose-phosphate backbone of DNA also acts as a hydrogen bond acceptor through the phosphate and sugar groups.
- Hydrogen bonds throughout DNA help in the stabilization of the double helix structure of the molecule.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- 1% – https://www.toppr.com/ask/en-np/content/story/amp/hydrogen-bonding-102367/
- 1% – https://www.sciencedirect.com/science/article/pii/S1631071304002780
- 1% – https://www.pnas.org/content/117/7/3592
- 1% – https://www.merriam-webster.com/dictionary/hydrogen%20bond
- 1% – https://www.easybiologyclass.com/difference-between-covalent-bond-and-hydrogen-bond-comparison-table/
- 1% – https://www.dictionary.com/browse/valence
- 1% – https://scienceinfo.com/covalent-bond/
- 1% – https://en.wikipedia.org/wiki/Chemical_bonds
- 1% – https://en.wikipedia.org/wiki/Carbon-hydrogen_bond
- 1% – https://en.wikipedia.org/wiki/Bond_polarity
- 1% – https://en.m.wikiversity.org/wiki/Covalent_bonding
- 1% – https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/04%3A_Covalent_Bonding_and_Simple_Molecular_Compounds/4.01%3A_Covalent_Bonds
- 1% – https://byjus.com/jee/hydrogen-bonding/
- <1% – https://www.vedantu.com/chemistry/hybridization
- <1% – https://www.sciencedirect.com/topics/chemistry/polar-covalent-bond
- <1% – https://www.britannica.com/science/chemical-bonding/The-hydrogen-bond
- <1% – https://studyqueries.com/polar-and-nonpolar-covalent-bonds/
- <1% – https://simple.wikipedia.org/wiki/Covalent_bond
- <1% – https://quizlet.com/344131455/chapter-8-flash-cards/
- <1% – https://quizlet.com/15964908/chapter-13-flash-cards/
- <1% – https://quizlet.com/119530563/anatomy-chapter-2-from-map-flash-cards/
- <1% – https://en.wikipedia.org/wiki/Talk:DNA/archive_1
- <1% – https://en.wikipedia.org/wiki/Hydrogen_bond

Hydrogen bonding is an intermolecular bond that occurs when there is a hydrogen atom in a molecule bonded to an electronegative and smaller element such as F, O and N.
https://science-hub.tech/what-is-a-hydrogen-bond-boiling-temperature/