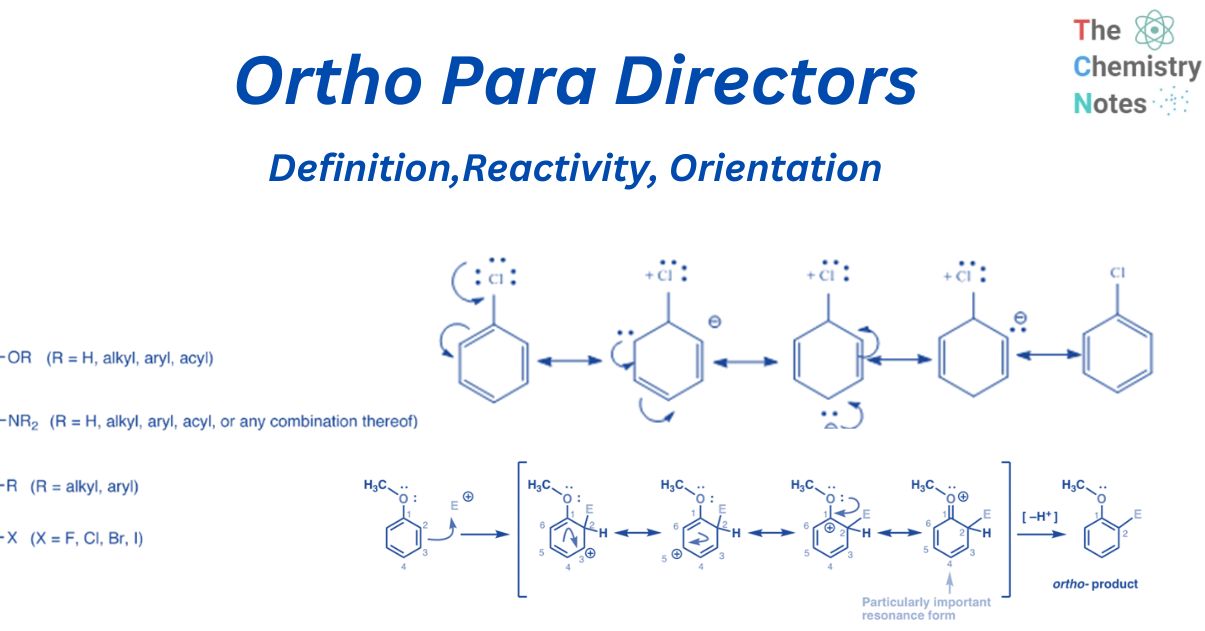
ortho para directors are electron donating groups on benzene that direct the electrophile E to the ortho- (1,2) and para- (1,4) positions. They are also known as ring-activating groups. For example, when aniline is chlorinated, the resulting products are ortho and para.
The entering group -Cl is directed to ortho and para positions on the ring by the (-NH2) group. As a result, the (-NH2) group is an ortho-para director. Some examples of ortho para directors include -Cl, -Br, -I, -OH, -NH2, -CH3, and -C2H5.
What are ortho para directors?
ortho para directors are substituent group that is already present in benzene and activates the ring for Electrophilic Aromatic Substitution processes in the ortho and para direction. Electron donors and activators are composed of a single pair of electrons or an electron density that “pushes” into the benzene. Substituents having a lone pair on the atom next to the aromatic ring are ortho para directors because they can create a new pi-bond with a neighboring carbocation.
Effect of ortho para directors on electrophilic substitution reaction
When mono-substituted benzene is electrophilically attacked, the rate of reaction and the site of attack differ depending on the functional group previously attached to it. Some groups, known as activating groups, boost the reactivity of the benzene ring, they are ortho para directors. Different reactions will have varying ortho and para percentages. Because of the steric barrier of its bulk, a bulky substituent will necessitate para addition.
As these negative groups prefer to be positioned exactly next to a carbocation, and the carbonation also occurs only in ortho and para addition, ortho para directors push entering electrophiles to add to the ortho or para position.
- When the atom directly linked to the ring (the key atom) in a monosubstituted benzene ring carries a lone pair of electrons, the electron density on ortho and para position is raised through conjugation (i.e., through the + R or + M effect). As a result, the incoming electrophile is connected to these regions, namely ortho and para. So they are ortho para directors.
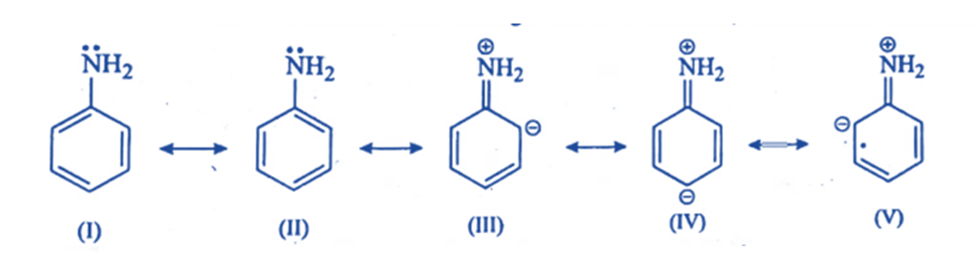
2. The electron density on ortho and para positions increases, and thus the overall electron density on the benzene ring increases, resulting in ring activation, i.e., the presence of ortho para directors (except halogens) facilitates further electrophilic substitution. E.g:
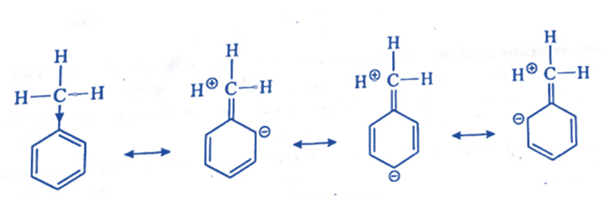
- The electron density on ortho and para positions of alkyl groups is raised by the + I and/or hyperconjugation effect. As a result, they are the ortho para directors and activating groups.
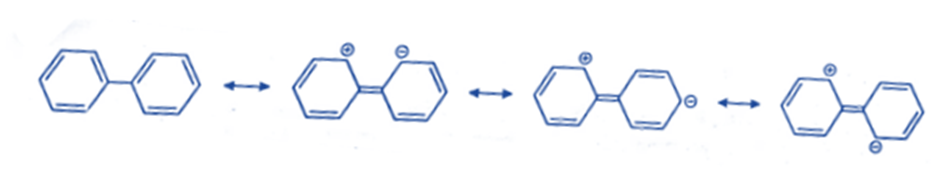
Aryl groups are also ortho para directors and activating groups because they raise electron density on the o- and p-positions in the following ways:
Electrophilic substitution on benzene with oxygen-containing Ortho, para directors
Although oxygen is electronegative the substituent group with oxygen directly attached to the benzene ring are ortho para directors. For example, benzene with -OCH3 group,
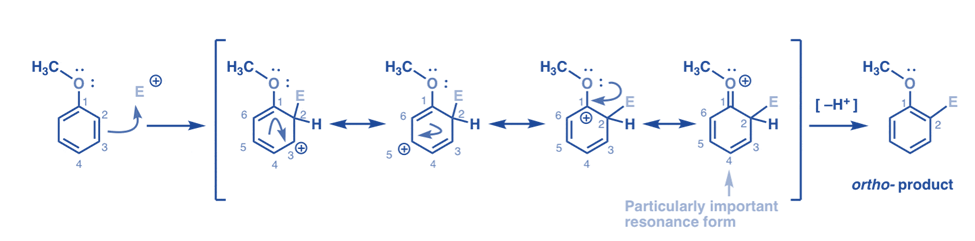
Ortho attack of electrophile
There are four types of resonance. Three of them have full carbocations (on C1, C3, and C5), but the fourth one – the resonance form with a C=O pi bond is of great importance. All of the carbon atoms in this resonance form have a full octet of electrons. Because the oxygen directly linked to the ring can give a single pair to the adjacent carbocation, a pi bond is formed. This is an illustration of pi donation.
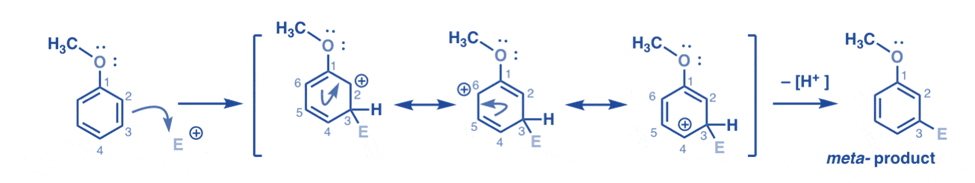
Meta attack of electrophile
The electrophile attack at C-3 produces a carbocation, which can be delocalized by resonance to C2, C4, and C6. However, there is no mechanism to “move” the carbocation to C1, implying that no acceptable resonance form exists in which all atoms have full octets.
As a result, the meta-carbocation intermediate is significantly less stable than the ortho-carbocation intermediate.
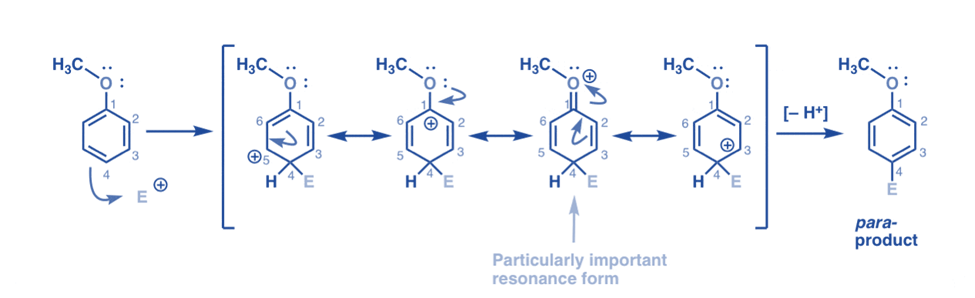
Para attack of electrophile
In this attack, we can draw resonance forms with carbocations on C1, C3, and C5, as well as a fourth resonance form where the linked oxygen atom gives an electron pair to the carbocation on C1, resulting in a full octet at carbon, in this attack. This is roughly the same as the ortho-intermediate condition.
Therefore, intermediate carbocations coming from ortho- and para-addition are significantly more stable than the intermediate from meta-addition, according to examination of the resonance forms.
Electrophilic substitution on benzene containing alkyl group
Although there are five hydrogens available for replacement on the benzene ring of toluene, two of them are ortho to the methyl group. Attacking from either of these positions yields the same result. The meta position is subject to the same considerations.
As a result, just three items are obtained. The actual distribution of these products reveals that just a small portion of the meta product gets received. Instead, the ortho and para isomers account for nearly all of the product. As a result, the methyl group is known as an “ortho-para directing group.” It “directs” the entering electrophile to attack in the ortho and para positions concerning itself. For example, nitration of toluene.
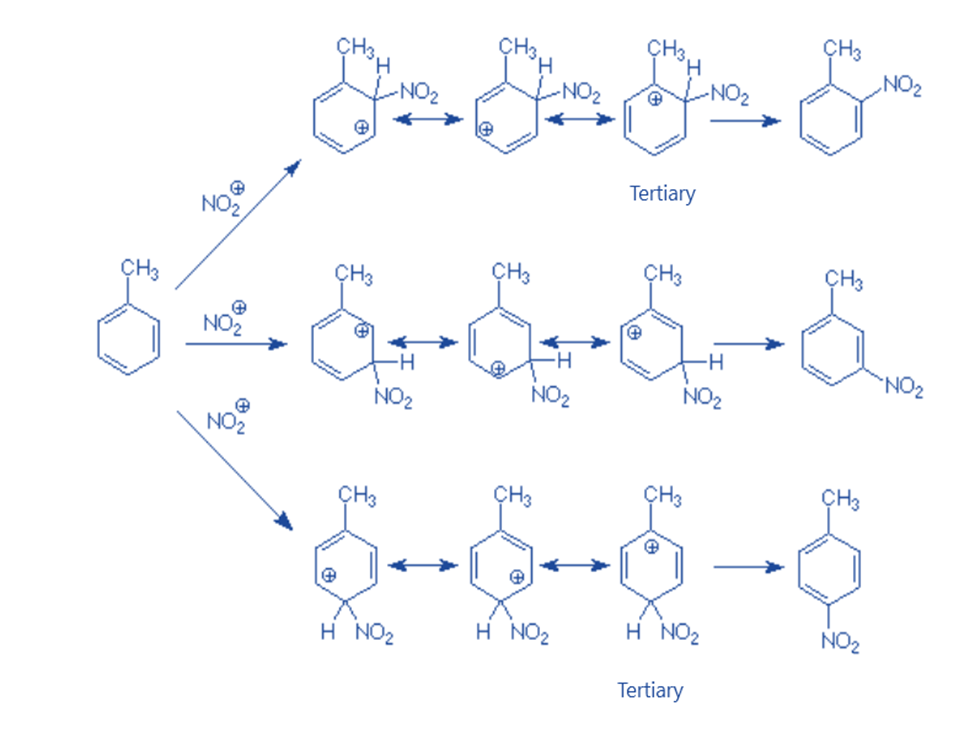
A resonance hybrid with three carbocations defines each intermediate. On the other hand, one of the contributing carbocationic structures for the intermediates resulting in the ortho and para products is tertiary. Because the electrons on the methyl group can directly stabilize the electron-deficient carbocationic carbon, this structure is more stable than the others. The resonance hybrid inherits this stability, making the intermediates for the attack more stable than those for the attack at the meta position.
Electrophilic attack will be faster at positions where the carbocations formed have positive charges on carbons bound to electron-donating groups like methyl groups. This happens when an electron-donating group is attacked at ortho and para places. So alkyl groups are ortho para directors.
ortho para directors, ring deactivating groups
Although halogens (F, Cl, Br, and I) are ortho, para directing, they deactivate the benzene ring, making additional electrophilic substitutions impossible. This is due to the existence of two opposing effects, namely the + R (or + M) and —I effects.
They are deactivators because they extract electrons from the ring inductively more strongly than they supply electrons via resonance. Inductive electron withdrawal deactivates all ring locations, however, electron donation by resonance partially compensates for this deactivation at the ortho and para positions. The meta is the most inactive state since it cannot benefit from resonance electron donation. As a result, the halogens act as ortho/para directors and deactivators.
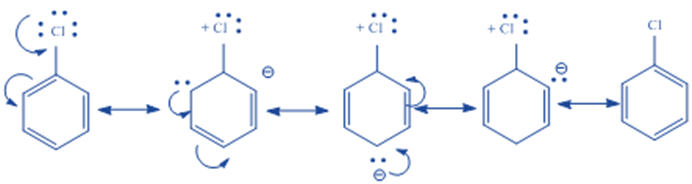
Based on the —I effect (F > Cl > Br >I) and + R effect (F< Cl< Br< I), one could expect the reactivity order to be reversed. The resonance effect does not follow the expected order based on relative electronegativity in this case.
This is owing to a difference in the size of the overlapping 2p orbital of the ring carbon and the p-orbital of the halogen because the overlap is greatest when the overlapping orbitals are comparable in size. Cl has a 3p orbital, Br has a 4p orbital, and I has a 5p orbital, all of which are larger than carbon’s 2p orbital, resulting in a very weak + R effect. However, because F and carbon have 2p-orbitals of comparable size, there is a larger overlap, resulting in a greater + R influence than Cl, Br, or I.
Good overlap with a carbon p orbital
There is a good overlap of p orbitals, but F is excessively electronegative.
Because fluorine is so electronegative, the inductive effect dominates over the resonance effect, making the ring more electron-deficient.
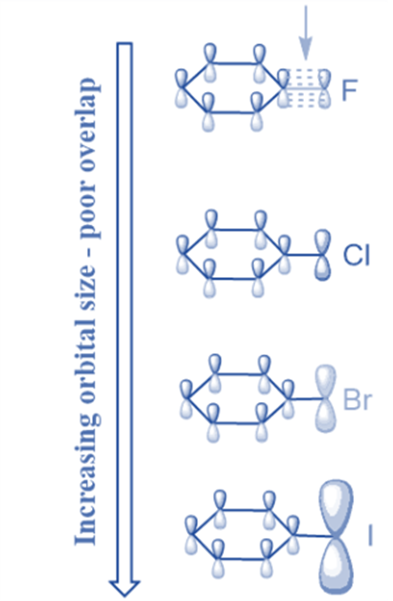
Poor overlap with a carbon p orbital
Cl, Br, and l are on the third, fourth, and fifth rows of the periodic table, respectively, and have little overlap with the carbon p orbitals, the orbital overlap reduces and the ring is deactivated.
The resonance effect is still strong enough to keep the sigma complex stable in electrophilic aromatic substations. As a result, halogens are deactivators, but ortho and para directors.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
