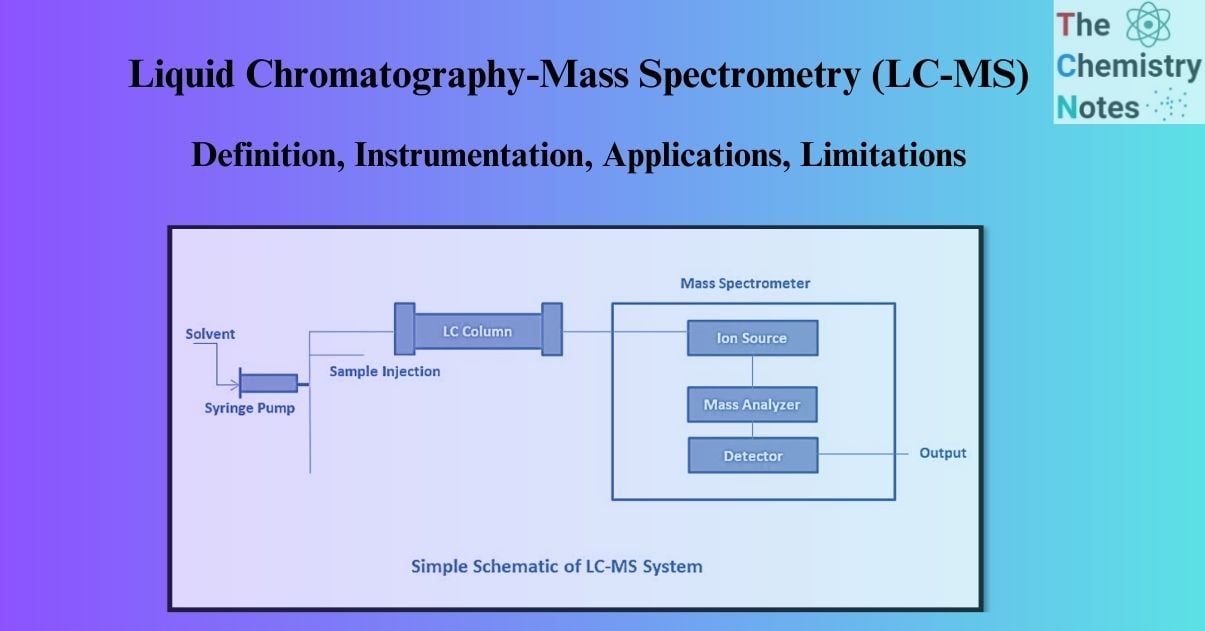
LC-MS (Liquid chromatography-mass spectrometry) is a highly sensitive and specific analytical method. Liquid chromatography coupled with mass spectrometry is known as LC-MS. In the presence of other components, Liquid Chromatography (LC) can be used to separate them out, and then the resulting eluent samples can be passed to Mass Spectrometry (MS) for detection, identification, and mass determination. LC-MS is used for quantitative and qualitative analysis of pharmaceutical drug ingredients, intermediates, and related chemicals.
In recent years, Liquid Chromatography Mass Spectrometry (LC-MS/MS) has become an essential instrument for conducting routine bioanalysis in numerous laboratory settings. This technology has gained widespread recognition and is now considered indispensable. LC Mass Spectrometry is a highly effective analytical technique that is utilized for both qualitative and quantitative analysis due to its exceptional sensitivity. It has been widely adopted in various configurations and has demonstrated remarkable performance.
What Is LC-MS (Liquid Chromatography Mass Spectrometry)?
The analytical methodology of LC mass spectrometry involves the combination of chromatographic separation of analytes with their subsequent detection based on mass. The utilization of liquid chromatography-tandem triple-quadrupole mass spectrometry (LC-MS/MS) has become prevalent due to its high sensitivity, selectivity, and accuracy. This technique is optimal for detecting analytes at nanomolar or even picomolar concentrations, including drugs, drug and food metabolites, biomarkers of disease progression or drug efficacy, pesticides, food contaminants, markers of ecosystem stability, and natural product extracts.
Liquid chromatography-mass spectrometry (LC-MS) is a powerful analytical technique that combines two selective separation methods. This allows for the isolation, detection, and quantification of analytes of interest, even in highly complex mixtures. LC-MS achieves this by comparing the measured analytes against known reference standards. Liquid chromatography (LC) is used to differentiate compounds based on their physico-chemical properties, while mass spectrometry (MS) is used to differentiate compounds based on their mass-to-charge ratios. The mass spectrometer has the ability to function as the “LC detector” and can potentially detect the specific species corresponding to each chromatographic peak based on its distinct mass spectrum. This reduces the necessity to separate isotopic or mass-differentiated isobaric components through chromatography. LC-Mass Spectrometry is a highly effective analytical tool for separation, detection, and quantitation due to its ability to provide dual selectivity.

Currently, the LC-MS/MS technique, also known as liquid chromatography tandem triple-quadrupole mass spectrometry (MS/MS), is the most commonly employed bioanalytical approach for quantification purposes. The LC/MS/MS methodology exhibits similarities to HPLC/UV in terms of both sample preparation and chromatographic configuration. In contrast to UV detection, MS/MS provides enhanced sensitivity and selectivity, enabling the analysis of larger sample sizes with lower quantitation limits. The employment of LC/MS/MS is a valuable, resilient, and highly responsive methodology utilized for a diverse range of low molecular weight compounds. Additionally, this particular technology is capable of being automated and analyzed without human supervision.
Principle of LC-MS
The LC-MS technique employs a High-Performance Liquid Chromatography (HPLC) system to fractionate the constituents of a mixture, which are subsequently subjected to ionization and partitioning based on their mass-to-charge ratio. Subsequently, the isolated ions are guided toward a detector, such as a photoelectric or electron multiplier tube, that recognizes and measures the concentration of individual ions. The ion source holds significant importance in mass spectrometry analysis, as it facilitates the effective production of ions for analytical purposes. Several ion sources, including APCI (Atmospheric Pressure Chemical Ionization) and ESI (Electrospray Ionization), are commonly utilized to ionize intact molecules. The selection of the ion source is contingent upon the chemical characteristics of the target analyte, specifically its polarity or non-polarity.
This technology offers significant benefits such as heightened sensitivity, specificity, and precision, as it enables analysis to be conducted at the molecular level. Furthermore, the structural characteristics of the analyte can be elucidated.
Mass spectrometry detection with liquid chromatography
Despite the availability of various detectors with different sensitivities and technologies for analyzing diverse sample types when combined with LC, the mass spectrometer (MS) has emerged as a highly selective, sensitive, and universally applicable detector that provides enhanced selectivity, sensitivity, and efficiency.
- Although various detector types facilitate flow-through analysis, it is not feasible for the LC eluent containing the analytes to flow directly into the mass spectrometer. The interface, also known as the ion source, serves as the coupling mechanism between the LC MS system and the mass spectrometers. The LC is operated under typical back-pressure conditions.
- The detector of the mass spectrometer is utilized in a vacuum environment.
- Upon the flow of the column eluent, the solvent undergoes ionization or evaporation through the application of voltage and heat. Subsequently, the analyte molecules carrying an electric charge are introduced into the interface. This step is crucial since the ionized particles are the only ones that can be detected and quantified by the mass spectrometer.
- The generation of analyte ions at atmospheric pressure within the interface is commonly referred to as Atmospheric Pressure Ionization (API), and the interface itself is recognized as the API source. Electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) are the predominant ionization sources utilized in the field of Liquid Chromatography-Mass Spectrometry.
- The ions of interest are attracted toward the mass spectrometer and subjected to the influence of both magnetic and electric fields. By altering the applied fields, the trajectories of ions undergo a transformation, resulting in their separation from one another on the basis of their mass-to-charge ratios.
- The accumulation and detection of ions can be performed by diverse mass detectors subsequent to separation. The electron multiplier is widely utilized as the primary mass detector. Upon collision with the surface of the electron multiplier, the detached ions prompt the emission of secondary electrons. This process occurs on a dynode.
- The multiplication of these secondary electrons is achieved by passing them through a series of dynodes.
- The current amplification resulting from the secondary electron flow is quantified and correlated with the ion intensities detected by the mass spectrometer at a specific moment in time.
- Integrated software modulates and tracks rapid hardware changes, while reported mass-to-charge ratios are determined based on the electromagnetic fluctuation in each of the quadrupoles as ions reach the detector, enabling differentiation.
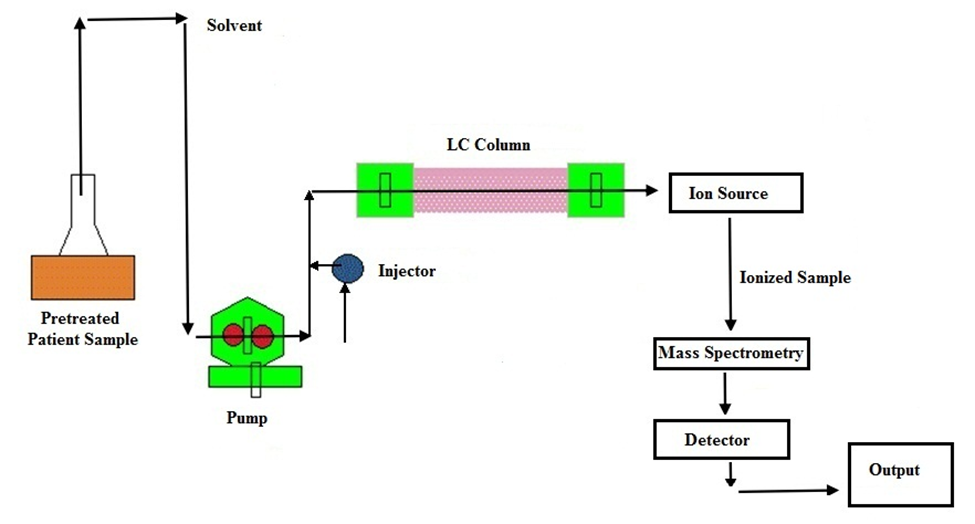
Figure: Schematic representation of LC-MS
(Source: https://b2capi.thyrocare.com/Liquid-Chromatography.html)
The Liquid Chromatography-Mass Spectrometry (LC-MS) technique is a hybrid of Liquid Chromatography and Mass Spectrometry that offers the separation capabilities of High-Performance Liquid Chromatography (HPLC) coupled with the detection capabilities of Mass Spectrometry (MS). Therefore, the two basic instrumentation components are:
- Liquid chromatography
- Mass spectrometry
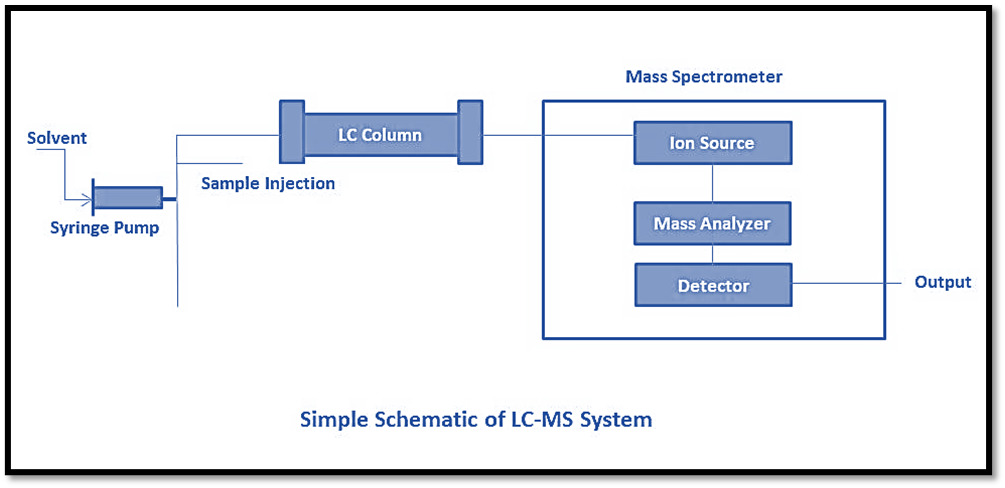
Liquid chromatography
Liquid Chromatography (LC) is a form of high-performance liquid chromatography that enables the separation of mixture components through the utilization of a liquid mobile phase and a solid stationary phase. Various forms of chromatography exist, including normal phase liquid chromatography, reversed-phase chromatography, ion-exchange liquid chromatography, chiral separation, and affinity liquid chromatography. Through the utilization of various column packing techniques that exhibit high efficiency, it is possible to separate complex mixtures with minimal quantities. The components of High Performance Liquid Chromatography (HPLC) are enumerated as follows:
- Pump: The substance is composed of materials that exhibit no reactivity towards solvents or any combination of aqueous buffer and organic solvents. The instrument is capable of providing a significant quantity of mobile phase, reaching a maximum flow rate of 10 mL/min. Three primary categories of pumps are utilized, namely reciprocating pumps, syringe pumps, and constant pressure pumps.
- Sample injector: It is inserted into the chromatographic apparatus to supply it with a sample volume. The typical injection range for samples is between 1 and 100 iL. Injector loops allow for injection volumes as high as 2 mL. Automatic injectors and manual injectors are the two most common types. When compared to their manual counterparts, automatic injectors are easier to use, more precise, and more comfortable for the patient.
- Column: It is a stationary phase made up of a mixture of silica material and a carbon chain. The standard range for column length is between 50mm and 300mm. Octadecyl (C18), Octyl (C8), Cyano, Amino, and Phenyl packings are employed in HPLC columns. Different types of compounds require different types of columns.
- Detector and recorder: The HPLC detectors are the most crucial components. The detector’s signal can be captured as a peak, and the corresponding data can be saved in a computer program.
Mass spectrometry
Mass spectrometry is an analytical methodology that relies on the determination of the mass-to-charge ratio of ionic species associated with the analyte being studied.Mass spectrometry (MS) is a valuable tool for determining the molecular mass and elemental composition of an analyte, as well as providing detailed structural elucidation of the analyte.
Parts of the LC-MS System
There are two main parts of an LC-MS system: the ionization source and the interfaces. The many parts of a mass spectrometer are detailed below:
- Ionization Sources and Interfaces
- Mass Analysers
Ionization Sources and Interfaces
Liquid chromatography is a technique used to separate liquid components, typically consisting of methanol, acetonitrile, and water. The mixture of components in liquid form is introduced into the ion source of the mass spectrometer. The ion source operates under high vacuum conditions. Vaporizing liquid drops while preserving the mixture of components is challenging due to pressure differentials. Interfaces are utilized to address this issue. Various interface types utilized in mass spectrometry are outlined below.
Direct liquid Introduction (DLI):
DLI ionization is achieved through chemical ionization and reagent gas vaporization of the solvent. Both solvent systems, normal and reverse phase, have been utilized. Methanol/water and acetonitrile/water (up to 60% water) are commonly employed as reverse-phase solvents. Buffers containing salts are generally not recommended due to the risk of capillary plugging during heating. DLI operates through the combined effects of thermal energy and liquid flow rate. The flow rate of the liquid entering the interface is restricted. The analyte ions generated by thermal energy are subsequently conveyed to the ion source via a capillary inlet or pinhole diaphragm.
Atmospheric-Pressure Ionization (API):
Nebulization, evaporation, and ionization comprise atmospheric-pressure ionization (API). Electrospray ionization (ESI) and atmospheric-pressure ionization (APCI) are the main API methods. When a solvent containing a sample is fed through a thin capillary tube and nebulized in a large chamber, atmospheric pressure ionization (API) produces a mist of minute droplets. Ionization occurs and certain droplets have a positive or negative electric charge. The solvent evaporates in massive heating chambers. Droplets shrink as the solvent evaporates. Ions and molecules collide. Ions entered the mass analyzer via capillary. The Atmospheric-Pressure Ionization (API) technique is utilized for a diverse array of moderate molecular weight analytes, both polar and non-polar in nature.
Electrospray Ionization (ESI):
Electrospray Ionization (ESI) involves passing the liquid sample through a stainless steel capillary tube that is held at a high positive or negative electric potential of approximately 3-5kV. Charged droplets are formed at the capillary tip and subsequently undergo vaporization. The evaporation of the solvent from droplets results in a reduction in their size and an increase in their surface charge. The collision ceases upon the conversion of highly charged droplets into gas phase ions. The gas-phase ions traverse the capillary sampling orifice and enter the ion source’s low-pressure region. The main benefit of ESI is that the ions are multiply charged, increasing by 1 to 3 for molecules 1000Da or over 50000Da. The m/z ratio is always below 2000. ESI-LC-MS measures the molecular weight of peptides, proteins, biological samples, polymers, nucleotides, sugars, and organometallics. Biomedical research and analysis also use it.
Atmospheric Pressure Chemical Ionization (APCI):
APCI involves two steps: analyte evaporation/desolvation and charged transfer reactions in the vapor phase to produce vapor phase ions. The Atmospheric Pressure Chemical Ionization (APCI) method involves nebulizing a liquid sample-containing solvent through a narrow capillary tube into a large chamber. Solvent evaporation occurs at atmospheric pressure in a sizable heating chamber, resulting in the formation of small droplets. Ionization occurs. Ionization typically occurs at temperatures ranging from 250 to 400 °C. The ions transfer charges to molecules via chemical reactions. The ions are passed through a capillary orifice in the mass analyzer. This technique is commonly employed for moderate molecular weight analytes with low polarity or non-polarity.
Thermo spray and Plasma spray Ionization (TSPI):
Thermo spray involves heating a capillary tube through which a liquid sample solution is passed, resulting in solvent evaporation. Charged droplets are created. The droplets decrease in size as a result of solvent evaporation. The surface charge density of droplets increases. The ions are subsequently introduced into a mass analyzer equipped with an electrostatic voltage system. Plasma spray does not produce ions, although corona discharge or plasma can enhance ions in thermal spray. Electric discharge ionizes neutral substances more. Enhanced molecular ionization. Clinical and medical analysis uses plasma spray because it is more sensitive.
Atmospheric pressure photo Ionization (APPI):
APPI utilizes photons to ionize and excite molecules. APPI involves two primary stages: exciting and ionizing the analyte from the eluent. APPI uses LC eluent vaporization like APCI. APPI uses Kr lamp for photon production. Kr lamp excites and ionizes molecules with high energy photons. Energy range minimizes analyte ionization. Ionized analytes go into mass analyzer. This methodology is advantageous for analytes that possess non-polar characteristics, rendering them challenging to ionize through Electrospray Ionization (ESI) and Atmospheric Pressure Chemical Ionization (APCI) techniques.
Particle Beam Ionization
The Browner et al. have devised a particle beam interface that enables the separation of solvents from solutes with minimal solute loss. The nebulization and evaporation processes bear resemblance to Thermo spray (TSP), Atmospheric pressure chemical ionization (APCI), and Electrospray ionization (ESI). The eluent obtained from HPLC or LC is passed through a narrow tube for separation. Helium gas injection generates high-velocity liquid droplets spray. The nebulizer’s liquid droplets undergo evaporation in the heating chamber, resulting in a reduction in their size. The liquid droplets exit the heating chamber as a particle beam. The beam subsequently traverses an ionization chamber, analogous to Electro Spray Ionization (ESI) and Atmospheric Pressure Chemical Ionization (APCI).
Continuous Flow Fast Atom Bombardment (FAB):
The FAB is a technique for interface detection that is both simple and highly sensitive. Fast atoms, such as Argon (Ar) or Xenon, are used to bombard the target in FAB liquid. The sample is dissolved in glycerol and applied onto a thin metal plate or probe. The probe is introduced into the mass spectrometer where ionization of the samples occurs upon bombardment by a beam of fast-moving atoms. The resulting ions are subsequently analyzed based on their mass-to-charge ratio (m/z). FAB is employed for the analysis of bulky and thermally labile molecules. It is utilized for surfactants and proteins.
Mass Analyzer
Following ionization, the ions are directed to a mass analyzer where they are separated based on their m/z ratio. Mass analyzers are typically evaluated based on their speed, time, rate, and reaction. The major components of mass analyzer are discussed below:
Quadrupole mass analyzer:
It is the most popular and practical mass analyzer available today. It has two rows of parallel rods between an ion source and a detector. The mass analyzer, which can separate ions in space or time based on their m/z. The linear Quadrupole mass Analyzer has four parallel hyperbolic or cylindrical rods in a radial array. Opposite rods are charged with a DC potential and an oscillating RF voltage. DC and RF applied to rods stabilize ions of one m/z, which are transmitted to the detector. The rods discharge unstable m/z ions. Low-accelerating potential introduces ions to Quadrupole. As they trend through Quadruple filter, ions oscillate perpendicular to rod length. Applying DC and RF voltage at constant ratio drives m/z-carrying ions toward detector. DC/RF ratio determines resolution. The Quadrupole typically scans at 1000 m/z at <4000 m/z. Mass accuracy rarely exceeds 0.1 m/z due to unit mass resolution. RF values are usually 1-2MHZ. 1000V DC and 6000V RF are possible.
Time of flight analyzer
Time of flight is a versatile method for various ion sources and inlet systems. The system lacks a magnetic field and requires only basic electrostatic maintenance and calibration. The ions are extracted and accelerated by a voltage. The duration of travel along the drift or flight path is contingent on the ion’s mass and charge. The time of flight for single charged ions (z=1, m/z =w) is directly proportional to their mass. Lighter ions will reach the detector before heavier ions as they move towards it. Simultaneous detection of all ions is achieved through scanning. The mass range scanning is fast and suitable for high m/z values.
Ion trap mass analyzer:
The Ion Trap Mass Analyzer exhibits high resolution, sensitivity, and the ability to perform multiple product ion scans. The Quadrupole ion trap is a 3D ion trap. The apparatus comprises a cylindrical ring electrode subjected to a Quadrupole field. Two additional types of electrodes are end-capped electrodes. One electrode of the end cap features a solitary central aperture for the introduction of electrons or ions into the trap, while the other electrode has multiple apertures or holes for the passage of ions to a detector. The ion trajectories are stabilized by the presence of Helium bath gas in the trap. The collision occurs between ions and a helium bath gas. The motion of ions enhances the trapping efficiency of the analyzer. The mass spectrum is generated by selectively releasing ions from the trap based on their mass-to-charge (m/z) ratios.
Fourier Transfer Ion Cyclotron Resonance (FT-ICR):
The Fourier transform ion cyclotron resonance (FT-ICR) is a highly significant mass analyzer. The ions generated by the ionization source are subsequently introduced into the mass analyzer, where they undergo separation based on their respective mass-to-charge ratios (m/z). The ions introduced into the chamber are confined within circular trajectories. The acceleration of ions is facilitated by the combined influence of electric and magnetic fields. As a result of this phenomenon, the ions become stimulated and produce a current that varies over time. The ions are segregated based on their mass-to-charge (m/z) ratios while being trapped.
Detectors:
The detector is a crucial component of the mass spectrometer, as it generates an electrical signal that is directly proportional to the quantity of ions that impact it. After the ions have been passed through the analyzer, it is necessary to detect them and convert them into a signal. The following is a list of commonly utilized detectors.
Point Ion Collectors Detector:
The ion collectors are situated at specific points within the mass spectrometer. All ions are directed toward a singular detector location. The detection of ions may be captured through the measurement of electric current and subsequently documented for analysis. The magnitude of electric current is directly proportional to the influx of ions detected at a specific point on the ion detector.
Array detector:
An array detector is a planar arrangement of point collectors. The ions are detected by an array detector at a specific location or across a plane. The ions are separated based on their mass-to-charge ratio (m/z) and detected using a planar ion collector. An array detector is capable of detecting ions with varying masses simultaneously and spatially differentiated.
LC-MS Analysis
Large proteins and tiny molecules in a variety of matrices have both benefited from the widespread use of LC-MS for analysis. Real-world applications of this technology include the following: the determination of alkylphenol ethoxylates (APE), the quantification of genotoxic impurities in active pharmaceutical ingredients, the detection of twelve model compounds that represent specific classes of doping agents in athletes (such as anabolic agents and stimulants) in exhaled breath, the detection of contaminants in food materials and dietary supplements, and the quantification of drug metabolites in biological samples.
Application of LC-MS
The combination of liquid chromatography (LC) and mass spectrometry (MS) is a commonly employed analytical technique in sample analysis, known as LC-MS. The high sensitivity of modern mass spectrometry has facilitated the replacement of multiple immunoassays by liquid chromatography-mass spectrometry (LC-MS). The utilization of LC-MS has led to enhanced efficacy in the process of drug discovery, owing to its exceptional specificity and sensitivity. The integration of the method with stable isotope dilution enables accurate and consistent measurements.
Biomedical applications:
The employment of the LC-MS methodology proves to be advantageous in identifying steroid drugs present in bodily fluids, as well as in creating a comprehensive analysis of endogenous steroids. LC-MS coupled with laser desorption and thermospray was employed for the initial analysis of amino acids. The molecular weights of nucleosides, nucleotides, saccharides, peptides, and proteins were analyzed through the utilization of LC-MS coupled with electrospray. Bile acids have been analyzed via liquid chromatography-mass spectrometry and thermospray ionization.
Environmental applications:
The utilization of LC-MS is prevalent in the examination of a wide range of samples, including but not limited to soil, drinking water, wastewater, air, and sludge. The specimens under consideration may pertain to a diverse array of chemical entities spanning from apolar hydrocarbons to ionic organometallic moieties. LC-MS can be employed to analyze a variety of pesticides and herbicides, such as triazine derivatives, chlorophenols, phenoxyalkanoic acids, and sulfonylurea herbicides.
Biochemical screening:
Metabolic disorders in newborns are detected through the analysis of blood samples using LC-MS. The utilization of second-tier liquid chromatography-mass spectrometry (LC-MS) analysis has been employed to corroborate the outcomes of primary immunoassays in neonatal screening.
Pharmaceuticals:
The application of LC-MS is prevalent in the identification of medicinal substances, with a particular emphasis on the isolation of chiral pharmaceuticals. Thermospray has been utilized to investigate antibiotics and prospective antimalarial agents. The successful demonstration of the application of LC-MS in the identification of bromazepam and analogous substances in cases of intoxication has been reported. The use of LC-MS in the detection, isolation, and purification of drug metabolites is a significant area of study. This is due to the fact that drug metabolites are often chemically or thermally labile, necessitating the use of liquid chromatography.
Vitamins and related metabolites:
The utilization of LC-MS has been widely favored as a technique for quantifying vitamin D and its metabolites. Assays utilizing liquid chromatography-mass spectrometry (LC-MS) have been devised for the detection and quantification of 25-hydroxyvitamin D2 and D3 in both plasma and serum samples. Assays of comparable nature are also accessible for the fat-soluble vitamins, namely vitamin K15 and Vitamin E13,15.
Steroid hormones:
The application of LC-MS analysis has proven to be advantageous in the realm of steroid biochemistry research, particularly in cases where conventional immunoassays have demonstrated limited efficacy. Sensitive LC-MS assays have been developed to quantify low levels of dihydrotestosterone and testosterone in females and minors.
Advantages of LC-MS
- The primary benefit of utilizing LC-MS lies in its exceptional analytical capabilities, which afford heightened sensitivity and selectivity, thereby enabling the precise determination of molecular weight across a diverse array of samples.
- The main advantage of utilizing LC-MS lies in its exceptional analytical capabilities, which afford heightened sensitivity and selectivity, thereby enabling the precise determination of molecular weight across a diverse array of samples.
- In a complex mixture, it can be used to isolate and identify solutes at concentrations as low as a few parts per million (PPM).
- The effective use of LC-MS is prevalent in various industries such as pharmaceuticals, bio-pharmaceuticals, research, forensics, food, and environmental sectors for the purpose of regulatory compliance.
- Compared to other chromatography techniques, the LC-MS method provides superior selectivity, resolution, precise mass measurement, and specificity.
- This method is unlike anything in its ability to detect and analyze unidentified constituents within a given sample solution.
- The implementation of this technology has the potential to optimize the research and analysis procedures in pharmaceutical manufacturing, as well as ensure adherence to regulatory protocols.
Limitations of LC-MS
- One of the primary limitations associated with LC-MS is its reliance on volatile buffers, which are necessary to prevent fouling of the API interface.
- Contamination: Contaminants can interfere with analysis by overlapping, suppressing, or enhancing analyte ionization, forming adducts with analytes, altering ionization potentials, masking analyte peaks, changing signal intensities, or appearing as ghost peaks in chromatograms. These effects can result in noisy baselines, system fouling, failure to meet analytical specifications, and contamination of the LC column, necessitating frequent maintenance and part replacement.
- One of the drawbacks of LC-MS is that it requires ionization of residual impurities for analysis.
- Knowledge and expertise are essential for conducting operations and analyzing data using liquid chromatography and mass spectrometry (LC-MS).
- Compared to other analytical instruments, the LC-MS incurs a relatively elevated cost of maintenance.
- The employment of LC-MS experiences substantial expenses, encompassing both initial investment and ongoing analytical expenditures.
- This particular instrument is not easily transportable and necessitates additional space.
References
- Johnstone RAW, HerbertCG (2011) Mass Spectrometry Basics, CRC Press Boca Raton London New, United Kingdom.
- Liquid Chromatography-Mass Spectrometry Third Edition Wilfried MA Niessen hyphen MassSpec Consultancy Leiden, The Netherlands © 2006 by Taylor and Francis Group, LLC, p. 32-81.
- Yergey AL, Edmonds CG, Lewis IAS, Vestal ML (1990) Liquid Chromatography/Mass Spectrometry: Techniques and Applications, © Springer Science+Business Media New York, USA, p.5-7.
- https://lupinepublishers.com/chemistry-journal/fulltext/liquid-chromatography-mass-spectrometry-and-its-applications-a-brief-review.ID.000103.php
- https://www.nebiolab.com/complete-guide-on-liquid-chromatography-mass-spectrometry-lc-ms/
- https://chrominfo.blogspot.com/2020/09/advantages-and-disadvantages-of-lc-ms.html
