Trace elements are any chemical elements found in trace amounts in the body. Trace elements can be categorized as potentially toxic, likely essential, or nutritionally essential. The nutritionally necessary components are necessary for proper metabolic and physiological processes. The human body contains at least 21 trace elements, each of which serves a different purpose. Any of the trace elements can affect a person’s growth and development and cause a variety of clinical manifestations.
Interesting Science Videos
What are Trace Elements?
The term trace elements refers to chemical elements found in trace amounts in natural materials.
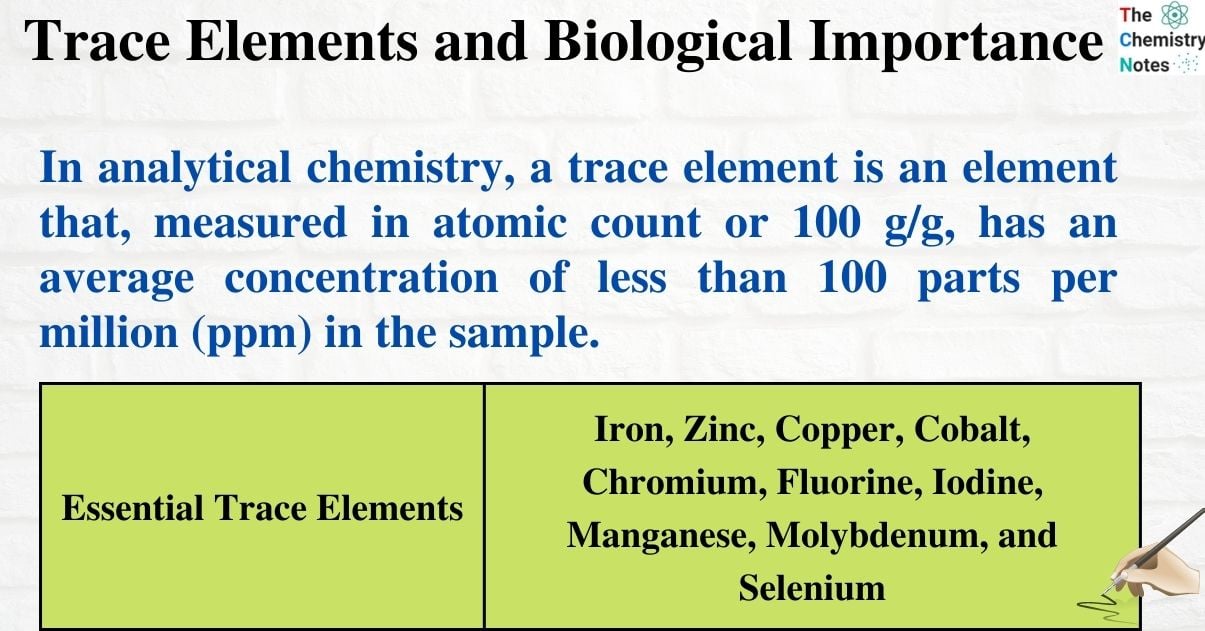
In analytical chemistry, a trace element is an element that, measured in atomic count or 100 g/g, has an average concentration of less than 100 parts per million (ppm) in the sample.
In biochemistry, a trace element is a dietary mineral that is required in extremely small amounts for the proper physiology, growth, and development of the organism.
The primary role of trace elements in enzyme systems is as catalysts, though some metallic ions, like iron and copper, also take part in oxidation-reduction processes during the energy metabolism process. Iron plays a crucial role in the transport of oxygen as a component of hemoglobin and myoglobin.
If consumed for long enough at high enough levels, all trace elements are toxic. For some essential trace elements, the difference between toxic intakes and optimal intakes that satisfy physiological needs is significant, whereas it is much smaller for other elements.
Classification of Trace Elements
The human body requires trace elements to maintain normal, complex physiological processes related to growth and development.
Depending on their biological impact, diseases caused by a deficiency, and toxicity from an overdose, these are classified as essential, probably essential, or non-essential.
| Essential Trace Elements | Iron, Zinc, Copper, Cobalt, Chromium, Fluorine, Iodine, Manganese, Molybdenum, and Selenium |
| Probably Essential Trace Elements | Nickel, Tin, Vanadium, Silicon, Boron |
| Non-essential Trace Elements | Aluminum, Arsenic, Barium, Bismuth, Bromine, Cadmium, Germanium, Gold, Lead, Lithium, Mercury, Rubidium, Silver, Strontium, Titanium, and Zirconium |
Essential Trace Elements are further divided into two sub-groups
| Trace Element | Iron, Zinc and Copper |
| Ultra-Trace Element | Manganese, Selenium, Cobalt, Chromium, Fluorine, Iodine and Molybdenum |
Biological Importance of Trace Elements
Zinc (Zn)
The established recommended daily amount (RDA) for Zn is 8 mg/day for women and 11 mg/day for men.
Source: Zn is found in wheat, brown rice, oats, lentils, soybeans, dried peas, black-eyed peas, lima beans, walnuts, peanuts, cashews, brazil nuts, cheeses, any kind of liver, and animal flesh such as beef, lamb, chicken, turkey, and various fish and seafood.
Additionally, it is present as the affordable, bioavailable source of sulfate, citrate, or oxide in the majority of vitamin-mineral supplements.
Function of Zinc
- Zn is a crucial trace element that is present in nearly 300 distinct enzymes and serves as a cofactor for some enzymes involved in metabolism and cell growth.
- Zn plays a role in the metabolism of proteins, carbohydrates, lipids, and energy as a component of numerous enzymes. It is essential for the proper operation of many bodily systems and is involved in numerous biochemical pathways.
- It is particularly crucial for healthy skin, as well as for a strong immune system and infection resistance.
- Zn is essential for protein and DNA synthesis, insulin activity, the metabolism of the ovaries and testes, and liver function, all of which are necessary for growth and cell division.
Deficiency of Zinc
Insufficient dietary intake of zinc may result in a deficiency. Nearly two billion people in the developing world are said to be zinc deficient . In many developing nations, zinc deficiency is a significant issue. Zn deficiency is the fifth most important risk factor for developing an illness, particularly pneumonia and diarrhea in children, which can result in high mortality rates in these underdeveloped areas.
- Stunted development in infants, children, and adolescents are two other severe deficiency symptoms.
- Early zinc deficiency also causes neuronal atrophy, behavioral issues, memory loss, impaired immune function, behavioral issues, and behavioral problems.
- In more severe cases, Zn deficiency results in hair loss, delayed sexual maturation, impotence, hypogonadism in men, and eye and skin lesions.
- It can also result in weight loss, sluggish wound healing, taste abnormalities, and mental lassitude.
Copper (Cu)
Cu is an essential trace element in plants and animals. The human body only contains about 150 mg of this vital mineral.
The established RDA for Cu in normal healthy adults is 2 mg/day.
Source: Wheat, barley, sunflower seeds, almonds, pecans, walnuts, peanuts, cashews, prunes, raisins, apricots, various dried beans, mushrooms, chicken, and most fish are the best food sources of copper for humans.
Function of Copper
- Many enzymes, including cytochrome oxidase, monoamine oxidase, catalase, peroxidase, ascorbic acid oxidase, lactase, tyrosinase, and superoxide dismutase (SOD), require copper as a component.
- Additionally, because it is found in so many different enzymes, Cu is a component of numerous metabolic processes. For instance, the SOD uses copper to help superoxide be converted to oxygen and hydrogen peroxide.
- A crucial micronutrient for the hematologic and nervous systems, copper (Cu) is essential.
- It also aids in the incorporation of iron into hemoglobin, the growth and development of bone, the development of myelin sheaths in the nervous system, the absorption of iron from the gastrointestinal tract, and the transfer of iron from tissues to the plasma.
Deficiency of Copper
Cu deficiency is uncommon in healthy adults, but it can happen in newborns.
- Fatigue, anemia, and a reduction in white blood cells are among the most typical signs of Cu deficiency.
- Nerve damage or osteoporosis can occur occasionally. Tingling and a loss of sensation in the hands and feet can result from nerve damage.
- Muscles might feel flimsy. Some people experience mild depression, irritability, and confusion.
- Due to the malabsorption of copper, it has been discovered that remote gastrointestinal surgery, such as gastric bypass surgery, is the most frequent cause of the copper deficiency.
- On the other hand, Menkes disease is a genetic Cu deficiency disorder with a wide range of symptoms and a high mortality rate.
Iron (Fe)
The most prevalent metal in a human body is Fe. The Fe content of the body is approximately 3-4 g nearly equals a concentration of 40–50 mg of iron per kilogram of body weight.
The established RDA for Fe in normal healthy adults is 8 mg/day for men and postmenopausal women and 18 mg/day for menstruating women (this is due to a lot of blood loss during their monthly period).
Source: Dietary Fe is abundant in red meat, liver, lentils, beans, peas, nuts, seeds, poultry, fish, shellfish, leafy greens, watercress, tofu, black-eyed peas, chickpeas, blackstrap molasses, fortified bread, and breakfast cereals. Additionally, molasses, teff, and farina all contain trace amounts of it. Fe in meat is easier to absorb than Fe in vegetables. Iron supplements can be used to treat anemia caused by Fe deficiency. Fe is typically found in vitamin/mineral supplements as common sulfates, fumarates, and gluconates.
Function of Iron
- Hemoglobin, an erythrocyte protein that transports oxygen from the lungs to the tissues, contains the majority of Fe in the body.
- The red color of blood is also a result of the Fe present in hemoglobin.
- The protein myoglobin, which transports oxygen to muscles, depends on fe.
- Fe is also required for development, normal cellular function, the production of some hormones, and the synthesis of connective tissue.
Deficiency of Iron
Fe deficiency is a condition caused when the body’s supply of readily available Fe is insufficient. The most prevalent nutritional deficiency in the world is a deficiency in fe.
- Fe deficient individuals are unable to produce enough hemoglobin to meet their body’s needs for oxygen transport.
- Fe-deficiency anemia is the diagnosis when the deficiency gets severe.
- The most typical signs of Fe-deficiency anemia are pale hands and eyelids as a result of low levels of oxygenated hemoglobin and fatigue and weakness brought on by insufficient oxygen delivery to the body’s cells.
- Aside from these, people may also experience fatigue, vertigo, hair loss, twitches, irritability, brittle or grooved nails, pagophagia, and restless legs syndrome.
- It has been noted that a low Fe level is typically linked to a higher risk of exposure to toxoplasmosis in females.
Magnesium (Mg)
After sodium and chlorine, magnesium is the third most common mineral in seawater and the eighth most common mineral overall. More importantly, it is the fourth most prevalent mineral in the human body and is required for over 300 bodily reactions. The average adult human body weighs about 25 g of magnesium.
With an RDA of 400 mg for healthy adult males and 320 mg for healthy adult females, magnesium is one of the ten essential minerals.
Source: The best food sources of dietary magnesium, according to some reports, are spinach, legumes, nuts, seeds, and whole grains. Additionally, it is discovered that foods high in magnesium include spices, nuts, cereals, cocoa, and vegetables.
Function of Magnesium
- Mg serves a variety of biological purposes, including it serves as a cofactor in over 300 enzymes, systems that control a variety of biochemical processes in the body, such as muscle, protein synthesis, and blood sugar regulation, nerve activity, and blood pressure control.
- Magnesium is needed for energy production. glycolysis, oxidative phosphorylation, and production.
- It is necessary for the synthesis of DNA, RNA, and the antioxidant glutathione and aids in the structural development of bone.
- It safeguards mitochondria which is the source of energy derived from harmful oxidants.
- It has been discovered that this mineral also contributes to the active transport of calcium and potassium ions across cell membranes, a procedure vital to the conduction of nerve impulses, the contraction of muscles, and a regular heartbeat.
Deficiency of Magnesium
Despite the fact that Mg deficiency is rare, it can happen, especially in those with poor diets or alcohol abusers. Additionally, taking certain medications (such as diuretics) can lead to magnesium deficiency.
- Loss of appetite, nausea, vomiting, fatigue, tingling or numbness, rapid heartbeat, delirium, hallucinations, sodium retention, low parathyroid hormone levels in the blood, and weakness are some of the early and moderate symptoms of magnesium deficiency.
- Inadequate Mg intake has been linked to cardiovascular disease, diabetes, high blood pressure, anxiety disorders, migraines, osteoporosis, and cerebral infarction, according to studies. It has also been shown to frequently cause muscle spasms.
- Additionally, it has been discovered that a severe magnesium deficiency can cause hypocalcemia or hypokalemia (low serum levels of calcium or potassium, respectively).
Manganese (Mn)
With an average concentration of 0.1%, manganese ranks as the 12th most abundant element in the crust of the Earth. The average human body has 12 mg of manganese. The rest is found in soft tissues like the liver, pancreas, kidneys, brain, and central nervous system. The skeletal system contains about 43% of it.
For adult males, the RDA for Mn is 2.3 mg/day and for adult females, 1.8 mg/day.
Source: It is known that a variety of dried beans and peas, nuts, seeds, wheat germ, whole grains (like buckwheat, bulgur wheat, rye, oats, brown rice, and corn), legumes, pineapples, tea, parsley, leafy greens, root vegetables (like sweet potatoes and beets), and sea vegetables are among the abundant dietary sources of Mn.
Function of Manganese
- Mn aids in the formation of bones, connective tissue, blood-clotting components, and sex hormones in the body.
- Additionally, it contributes to the metabolism of fats and carbohydrates, calcium absorption, and blood sugar control.
- Mn is also required for healthy nerve and brain function.
- Mn is also an essential part of enzyme systems, such as those that handle oxygen. It belongs to the antioxidant SOD, which aids in battling free radicals.
Deficiency of Manganese
Mn is essential for human survival, but when intake rises above the recommended level, health issues can result. The abnormal concentrations of Mn in the brain, particularly in the basal ganglia, have been linked to neurological conditions like Parkinson’s disease. For adults consuming Mn in their daily diets, the National Academy of Sciences set a tolerable upper intake level of 11 mg.
- However, it has been discovered that low levels of Mn (an Mn deficiency) can lead to hypercholesterolemia, impaired glucose tolerance, dermatitis, changes in hair color, skeletal abnormalities, infertility, deafness, and impaired synthesis of vitamin K-dependent clotting factors.
Mn can be found in a wide range of forms, including Mn chelates (aspartate, picolinate, fumarate, malate, succinate, citrate, and amino acid chelate) and Mn salts (sulfate and gluconate). Mn supplements can be taken as tablets or capsules, typically in the form of a multivitamin with other vitamins and minerals.
Nickel (Ni)
It is a naturally occurring element of the crust of the earth and is found in igneous rocks.
Dusts produced by volcanic eruptions and soil and rock weathering are two natural sources of nickel.
For nickel, no RDA has been established.
However, it has been reported that the daily intake of nickel from food and water is estimated to be between 80 and 130 g worldwide.
Source: Many foods contain ni, including beans, chocolate, soybeans, lentils, split green peas, oats, buckwheat, barley, and corn. The best sources of nickel are nuts like hazelnuts and walnuts. There is a moderate amount of nickel in many fruits and vegetables, including bananas and pears.
Function of Nickel
- It is necessary for urease synthesis and efficient urea metabolism.
- RNA, which contains the highest concentrations of nickel in the body, is thought to play some role in protein structure or function even though the biological function of nickel in the human body is still not entirely clear.
- Nickel is thought to function as a cofactor in the activation of specific enzymes involved in the breakdown or utilization of glucose.
- Ni may contribute to the synthesis of prolactin, which would then affect the production of human breast milk. The characteristics of this fascinating mineral in the human body still need to be explored.
Deficiency of Nickel
- Although Ni deficiency has not been demonstrated to be a problem in humans, it may still result in biochemical changes, such as decreased Fe resorption, which can cause anemia.
- It can cause damage akin to parakeratosis and interfere with the incorporation of calcium into the skeleton, which thus manifests as disturbed Zn metabolism.
- The metabolism of carbohydrates is particularly impacted by nickel deficiency, according to research. More studies are thus needed to determine the advantages of and potential health effects of a nickel deficiency in humans.
Chromium (Cr)
Humans only need trace amounts of the element Cr. The two main forms are trivalent (chromium III), which is biologically active and found in food, and hexavalent (chromium VI), which is a toxic form brought on by industrial pollution.
For adult males and adult females, the recommended daily intakes of chromium are 35 mg and 25 mg, respectively.
Source: Although Cr is widely distributed in the food supply, most foods only contain modest amounts. Egg yolks, whole grains, high-bran cereals, coffee, nuts, green beans, broccoli, meat, and brewer’s yeast are all sources of it.
Function of Chromium
- The amount of Cr in biological matter has been extensively researched. Chromium has been found to significantly increase enzyme activity, play a key role in carbohydrate metabolism, stimulate the liver’s production of fatty acids and cholesterol from acetate, and improve sugar metabolism by triggering insulin.
- Additionally, it has been discovered that chromium makes the tissues of the body more responsive to insulin.
- Therefore, it is a crucial cofactor in how insulin works.
Deficiency of Chromium (Cr)
In reality, very few people have chromium deficiency. Despite this, some studies found a link between insulin resistance and glucose intolerance in patients receiving long-term parenteral nutrition and chromium deficiency. Additionally, a lack of chromium has been linked to an increase in hematological parameters, including hemoglobin, hematocrit, erythrocytes, leukocytes, and mean erythrocyte volume.
Cobalt (Co)
Co is a crucial component of cobalamin, also known as vitamin B12 or cobalamin, which is an essential trace element for the human body.
The average daily intake of cobalt from food is 5 to 40 g, the majority of which is inorganic. The body is toxic to inorganic forms of cobalt, and the longer they remain there, the more damage they do to cells.
Source: Cobalt is most abundant in green vegetables and fresh cereals, while it is least abundant in dairy products, refined cereals, and sugar.
Function of Cobalt
- Co ions can enter the human body in a number of ways, including through food, the respiratory system, the skin, and as a component of biomaterials.
- Any of the aforementioned entry points allow cobalt ions to enter the body where they bind to proteins in the bloodstream and travel through the bloodstream to the tissues and cells where they are deposited.
- Additionally, it plays a significant part in the production of amino acids and neurotransmitters.
Deficiency of Cobalt
- Cobalt deficiency has been linked to disruptions in vitamin B12 synthesis.
- It may result in anemia, and hypothyroidism, and is also an elevated risk of failure and abnormal infant development.
- The overabundance of this metal in the human body may also result in hypothyroidism, excessive erythrocyte production, lung fibrosis, and asthma.
Lead (Pb)
Pb is a very prevalent metal in daily life. Some common sources of lead include water, paint, electric storage batteries, insecticides, and gasoline. By inhaling, ingesting, or coming into contact with the skin, it enters the human body and is quickly absorbed into the bloodstream. Lead is distributed throughout the body into three main compartments: blood, soft tissue (such as the kidney, bone marrow, liver, and brain), and mineralized tissue (such as bones and teeth).
Lead has no known health benefits or biological roles in the human body. Lead, on the other hand, has harmful effects that harm the human body.
Almost every organ and system in the human body is susceptible to it. It can severely harm the brain, kidneys, nervous system, and reproductive system, and thus can result in high blood pressure. It has been determined from these dysfunctions that the nervous system is the organ most susceptible to lead poisoning.
Pb causes serious disorders in young children and fetuses in particular. Lead toxicity occurs at blood levels of approximately 40–60 g/dL, even though there is no safe level of exposure to it.
Selenium (Se)
Se is a crucial micronutrient for both plants and animals. The average human body contains 13–20 mg of selenium.
It is a necessary mineral with an RDA of roughly 70 g/day.
Source: Seafood, meats, whole grains, and some vegetables are the best food sources for selenium. The selenium content of raw foods was found to be significantly higher than that of cooked and processed foods
Function of Selenium
- Se is a crucial trace element for maintaining the health of the human body. It is present as selenocysteine at the active site of a variety of selenoproteins.
- It is also a crucial part of antioxidant enzymes like glutathione peroxides and thioredoxin reductase.
- Se, on the other hand, appears to have a protective effect against some cancer types, including breast, prostate, and colon.
Deficiency of Selenium
- Even though selenium deficiency is uncommon in healthy people, it can be extremely toxic if taken in large doses. Dietary selenium has been shown to be crucial for a strong immune system because it improves T-lymphocyte immune responses.
- Low blood levels of selenium have been linked to a higher mortality rate from cardiovascular disease, according to research.
- Additionally, selenium deficiency has been cited as the primary cause of Keshan disease.
Iodine (I)
- Iodine plays a crucial role in the thyroid gland’s ability to produce T3 and T4, which are the two main thyroid hormones.
- Goitre is an enlargement of the thyroid gland that results from a diet low in iodine. Iodine is absorbed into the body as iodate(V) ions found in marine plants and animals or as iodine ions found in table salt, drinking water, and cow’s milk.
- Excess iodine is excreted from the body. When this pool is deficient in iodine, goitre can occur.
- Iodine-131 overdoses, such as those brought on by nuclear accidents, can be fatal.
Trace Elements and Health
For some enzymes involved in metabolism and cell growth, the majority of which are involved in the metabolism of proteins, carbohydrates, lipids, and energy, essential trace elements play a crucial role as cofactors. In addition, they are essential for cellular health, hormone production, muscle and nerve development, growth, and development.
Understanding the etiology of some diseases, such as cancer, may be made possible by understanding the role of trace elements in biological processing. The ability of trace elements to act as a significant influencer in a variety of life-sustaining processes, such as controlling homeostasis and preventing free radical damage, can explain the clear association between trace element content and many common diseases.
Learn out more
Reference
- Essential Trace Elements and Their Vital Roles in Human Body. Indian Journal of Advances in Chemical Science_ DOI: 10.22607/IJACS.2017.503003
- https://alevelnotes.com/notes/chemistry/elements-of-life/trace-elements
- https://en.wikipedia.org/wiki/Trace_element
- https://study.com/learn/lesson/trace-elements-overview-examples.html
- 1.X. Chen, G. Young, J. Chen, X. Chen, Z. Wen, K. Ge, (1980) Studies on the relations of selenium and Keshan diseases, Biological Trace Element Research, 2: 91-107
- H. Ensminger, J. E. Konlande, (1993) Foods & Nutrition Encyclopedia, 2nd ed. Boca Raton, Florida: CRC Press, p2368-2369
- J. Osredkar, N. Sustar, (2011) Copper and zinc, biological role and significance of copper/zinc imbalance, Journal of Clinical Toxicology

