Hydrochloric acid is an extremely corrosive inorganic chemical. It has the chemical formula HCl. Glauber created hydrogen chloride in 1648 by heating sodium chloride with pure H2SO4. Davy demonstrated in 1840 that HCl is a combination of chlorine and hydrogen. Hydrochloric acid is also known as muriatic acid.
What is Hydrochloric acid?
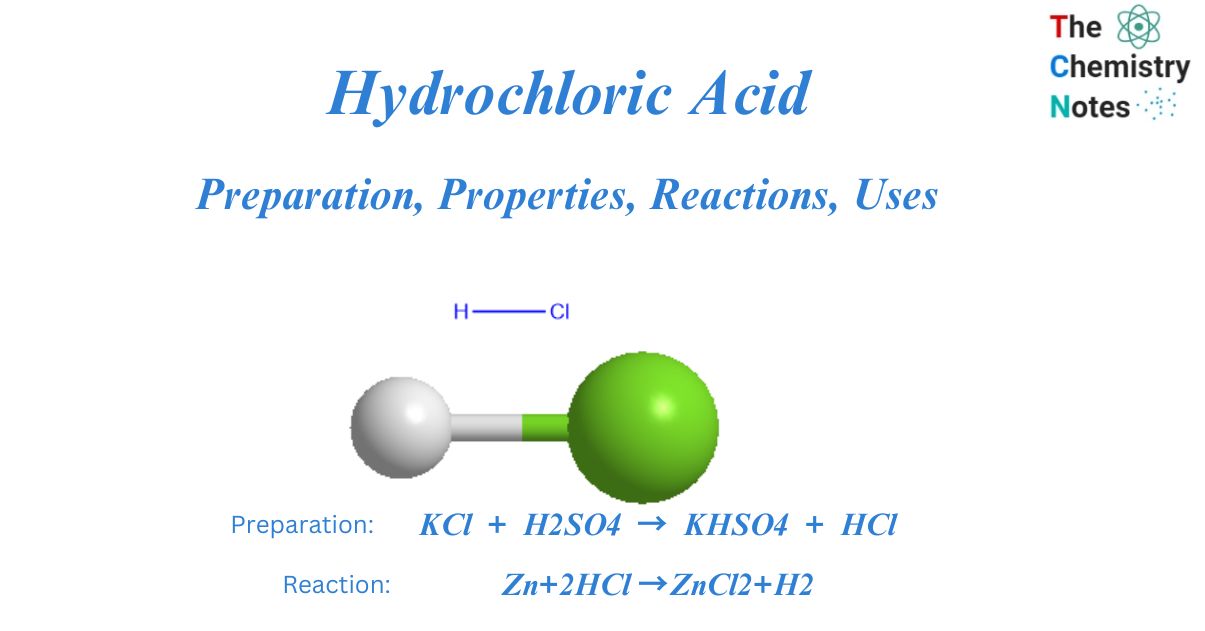
The chlorine atom and hydrogen atom form a single covalent bond in hydrochloric acid, which is a simple diatomic molecule. Because the chlorine atom is more electronegative than the hydrogen atom, the bond formed between them is polar.
HCl is acidic and has a characteristic unpleasant odor. It can be used as a laboratory reagent as well as in industry. Hydrochloric acid is used in the manufacture of gelatin and the tanning of leather. The physical characteristics, density, boiling point, melting point, and pH of HCl are determined by its molarity or concentration.
Preparation of Hydrochloric acid
General method
- A direct combination of elements can be used to produce hydrogen chloride:
In the presence of diffused sunlight, moist hydrogen, and chlorine can be combined to generate hydrogen chloride gas. Moisture catalyzes this process. Because the reaction is explosive in direct sunshine and relatively slow in the dark, it is carried out in diffused sunlight.
H2 +Cl2 → 2 HCl
- By reacting metal chloride with concentrated sulphuric acid
When metallic chlorides react with concentrated sulphuric acid, hydrogen chloride gas is produced.
KCl + H2SO4 → KHSO4 + HCl
Laboratory preparation
Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride with concentrated H2SO4. The gas can be dried by passing it through a sulfuric acid solution. Conc. H2SO4 is an appropriate drying agent for this purpose since it does not react chemically with the HCl gas. It just absorbs water. Other drying agents like phosphorus pentoxide (P2O5) and quick lime (CaO) cannot be used to dry HCl gas because they chemically react with it.
NaCl + H2SO4 → NaHSO4 + HCl
NaHSO4 + NaCl → Na2SO4 + HCl
The hydrogen chloride gas is collected by upward displacement of air. This is due to it being 1.27 times heavier than air. As it is very soluble in water, it is not gathered over water.
Other methods
Hydrochloric acid is made by dissolving hydrogen chloride and water. When hydrogen chloride gas enters water, water molecules pull the hydrogen atom in HCl(g) away from the chlorine atom. This is the dissolution process that results in Hydrochloric Acid.
HCl + H2O → H3O + Cl−
Hydrogen chloride is also produced as a byproduct of industrial-scale chemical synthesis. It is prepared industrially through the combustion of hydrogen and chlorine. Because of reparation, a high concentration of HCl is difficult to prepare. In large amounts, HCl can be manufactured commercially by the synthetic approach based on a direct combination of hydrogen and chlorine.
Physical Properties of Hydrochloric acid
- Hydrogen chloride is a colorless gas with a strong smell.
- It freezes at 114 °C (173 °F) and condenses at 85 °C (121 °F).
- Because of its high solubility in water, the gas fumes in moist air.
- In an aqueous solution, the chemical dissociates extensively into a hydronium ion (H3O+) and a chloride ion (Cl).
- Hydrogen chloride is extremely unreactive when completely dried.
- Gaseous hydrogen chloride reacts with active metals and their oxides, hydroxides, and carbonates to form chlorides.
Chemical properties of Hydrochloric acid
- Hydrogen chloride gas is neither combustible nor supportive of combustion. It extinguishes a flaming splint.
- When heated over 500oC hydrogen chloride dissociates into hydrogen gas and chlorine gas.
2 HCl → H2 +Cl2
- When hydrogen chloride gas is heated with various metals such as Zn, Mg, Fe, etc., corresponding metal chlorides are generated along with the evolution of hydrogen gas.
Zn+2HCl →ZnCl2+H2
Mg+2HCl →MgCl2+H2
Fe+2HCl →FeCl2+H2
Uses of Hydrochloric acid
- It is used as a laboratory reagent.
- This acid is used to clean table salt. It is also useful for regulating the acidity of solutions and controlling the pH of medicinal items, water, and foods.
- It can be used to create chlorides, which are Cl- -ion salts.
- It’s also used in titration to detect the amount of bases because it’s a strong acid that produces more precise results.
- Large-pore structures are created when hydrochloric acid is added to a rock. This has significantly aided the production of oil.
- Because of its highly corrosive nature, hydrophilic acid is used as a chemical for removing spots or rust from metals such as copper and iron.
- Dilute hydrochloric acid is used to remove rust or iron oxide from steel or iron before it is processed into wire, sheet and strip coating, and tin mill products.
- It is used for the production of organic and inorganic compounds.
- HCI is employed in the production of chlorine, aqua regia, and other chlorides.
- Hydrogen chloride is a solvent used to dissolve noble gases.
- It is used for tanning, refining, and manufacturing of leather.
References
- https://byjus.com/chemistry/hydrochloric-acid/#:~:text=Hydrochloric%20acid%20is%20an%20inorganic,is%20a%20simple%20diatomic%20molecule.
- https://www.toppr.com/guides/chemistry/acids-bases-and-salts/hydrochloric-acid/#:~:text=Hydrochloric%20acid%20preparation%20takes%20place,process%20which%20forms%20hydrochloric%20acid.
- https://www.vedantu.com/chemistry/hydrochloric-acid.
- https://unacademy.com/content/jee/study-material/chemistry/reaction-with-hydrochloric-acid/.
- https://www.britannica.com/science/hydrogen-chloride

Is this chemical found in any way ….in the tablet metformin..,…used as medication for diabetes type 2. ¿?¿??????
Metformin hydrochloride, a salt of metformin, is the active component of metformin. HCl is not a component of metformin tablets. The hydrochloride part of the compound is a chemical salt, not the free acid. It does not contain hydrochloric acid as a separate component.