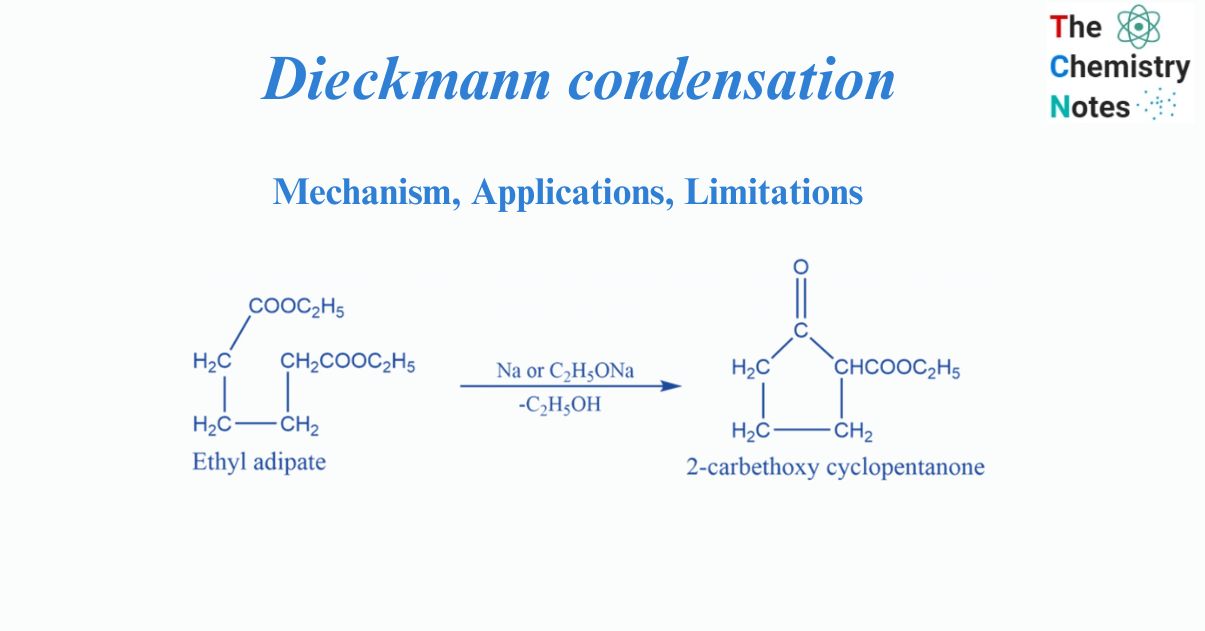
Dieckmann condensation involves the intramolecular condensation of diester in the presence of bases to produce ß-keto esters. It is named after the German chemist Walter Dieckmann.
The Dieckmann Condensation is effective for producing 5- or 6-membered cyclic ß-keto esters and is typically carried out with sodium alkoxide in an alcoholic solvent. If the product contains an enolizable proton, the yields are high.
What is Dieckmann condensation?
The Dieckmann condensation is an organic reaction that uses an alkoxide base in alcohol to generate a carbon-carbon bond between two ester groups. The end outcome is a cyclic β–ketoester. Dieckmann condensation is Intramolecular Claisen condensation. It is an organic process that occurs when an ester of C6, C7, or C8 dibasic acid undergoes intramolecular condensation in the presence of condensing agents such as sodium, sodium ethoxide, or potassium t-butoxide.

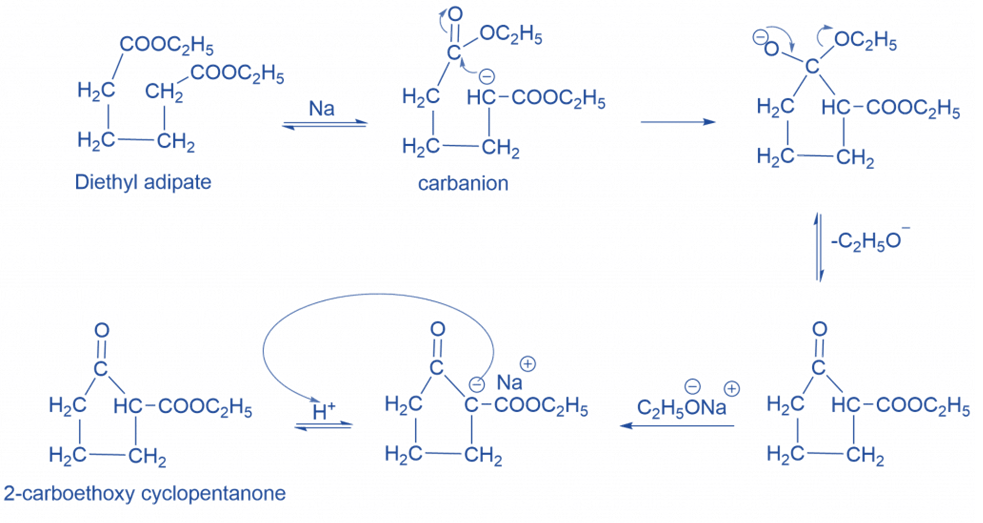
Mechanism of Dieckmann condensation
In this mechanism, the base deprotonates the ester at the α-position, resulting in a generation of carbanion, which subsequently attacks the carbon of the second ester group to produce cyclic enol. The synthesis of the β-keto ester is now caused by protonation.
Applications of Dieckmann condensation
- 5-, 6-, 7-, and 8-membered rings are produced in high yields by the Dieckmann condensation.
- Because of the steric stability of five- and six-membered rings, these structures will develop preferentially.
- It is used to create cyclic indole.
- It also played a role in the synthesis of five-membered pyrroles.
- A Dieckmann cyclization’s cyclic beta-keto ester product can be changed using reactions similar to those utilized in acetoacetic ester synthesis.
- The beta-keto ester’s acidic alpha-hydrogens allow it to be easily deprotonated and alkylated in an alpha-substitution process. The presence of a carbonyl group in the beta position enables the ester to be removed via decarboxylation. As a result, this procedure can be used to prepare 2-substituted cyclopentanones and cyclohexanones.
Limitations of Dieckmann condensation
- Esters of dibasic acids with fewer carbon atoms cannot proceed through Dieckmann condensation due to the high strain generated in the tiny rings.
- Esters of dibasic acids with more than eight carbon atoms do not undergo the Dieckmann reaction because the intramolecular reaction slows down as the chain length grows and the intermolecular condensation process is preferred.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
