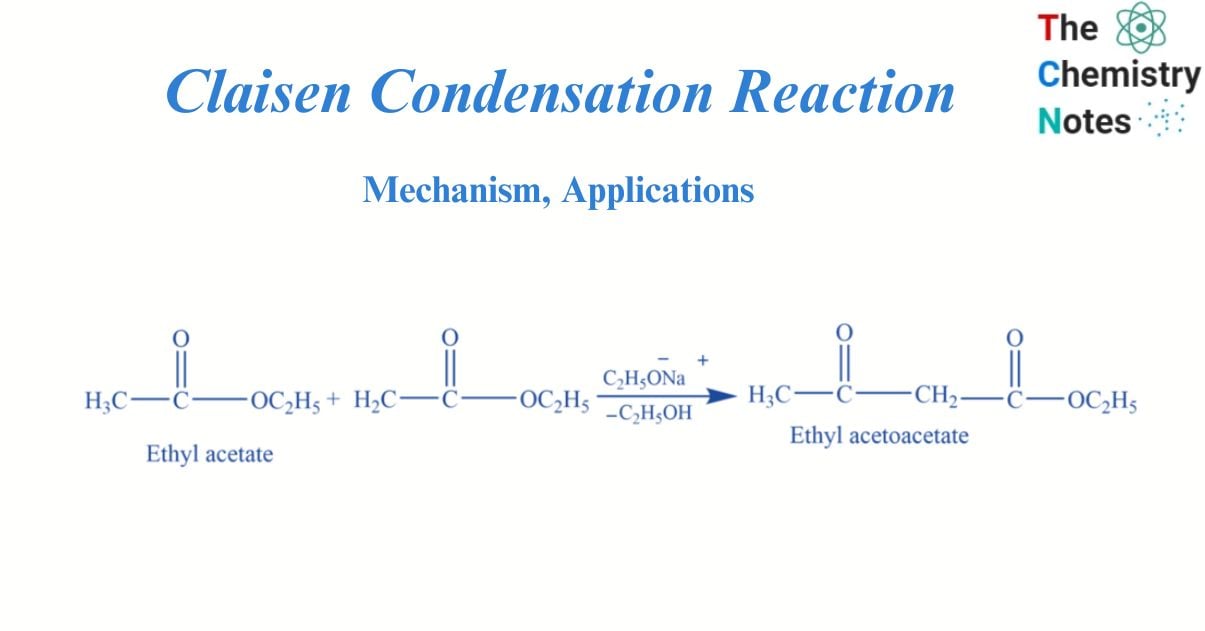
The Claisen condensation reaction involves the formation of a β-keto ester by the reaction of two molecules of esters with an α-hydrogen atom with a strong base like sodium ethoxide.
Water is produced as a byproduct of the condensation reaction. A strong base is present in this acyl substitution reaction between esters and a carbonyl molecule. In this process, the carbonyl of one molecule is combined with the enolate of another to create β -keto esters and alcohols.
Both molecules retain their carbonyl group in the Claisen reaction due to the presence of the alkoxy group, which acts as a leaving group after the nucleophilic addition.
This reaction was named after Rainer Ludwig Claisen, a German chemist.
It is a crucial method for the synthesis of β–ketoesters or β–diketones in organic chemistry and is frequently used for the synthesis of several compounds, such as medicines, flavorings, and fragrances.
Interesting Science Videos
What is Claisen condensation reaction?
In the presence of a strong base (C2H5ONa), esters with α hydrogen undergo a condensation reaction to form β keto ester. This is known as Claisen condensation reaction.

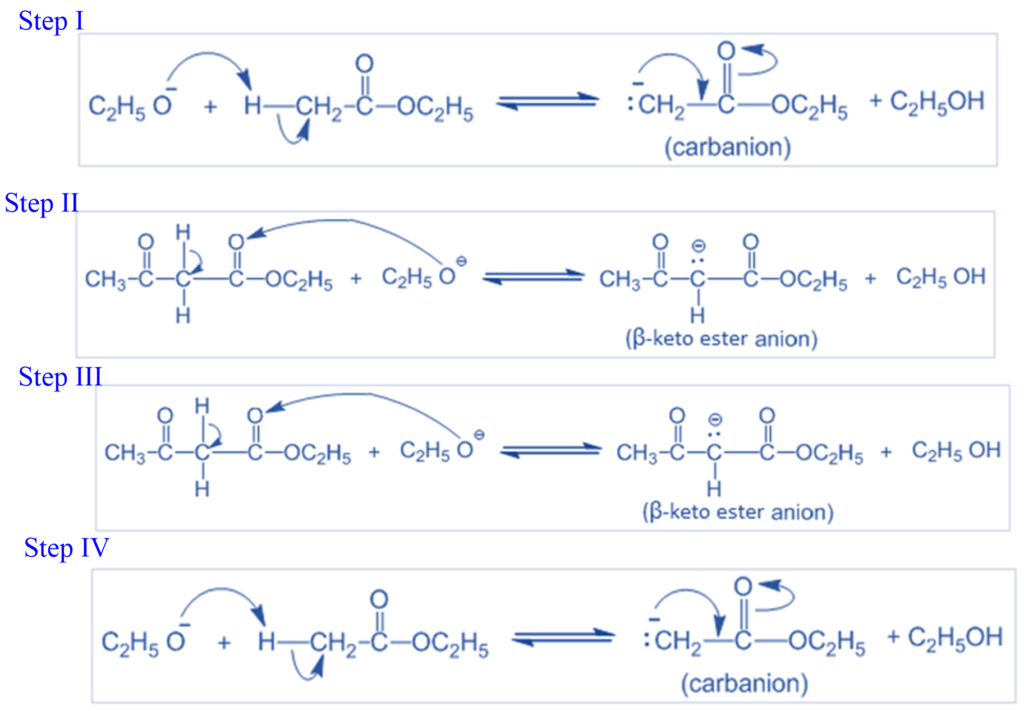
Mechanism of Claisen condensation reaction
- Step I: In this step, an ethoxide ion forms a carbocation by removing a proton from the α–carbon of one molecule of ethyl acetate.
- Step II: The ester’s C=O group is attacked by carbanion in a nucleophilic way, generating an anion that loses the ethoxide ion to produce β–ketoester. At this stage, the Claisen condensation varies from the aldol condensation. Nucleophilic attack results in addition to the aldol condensation and substitution in the Claisen condensation reaction.
- Step III: Ethoxide ion and β-keto ester undergo an acid-base reaction in this stage, resulting in β-keto ester anion and ethanol.
- Step IV: This step involves adding an acid to the reaction mixture to give the β-keto ester anion, forming the β-keto ester that is in equilibrium with its enol form.
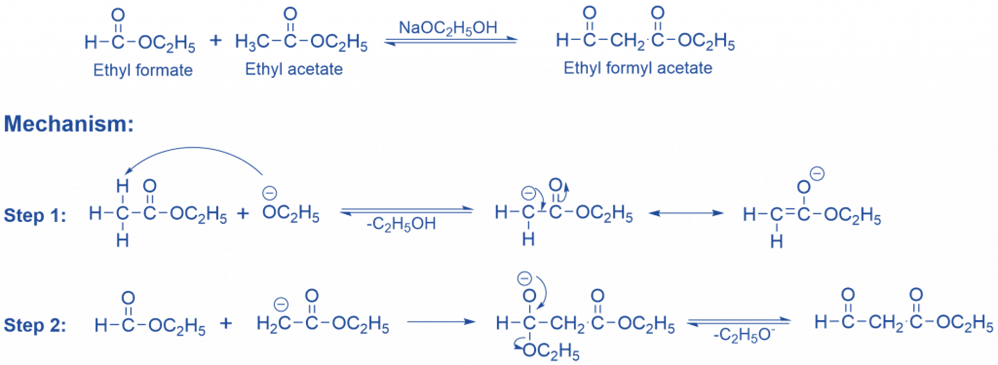
Cross Claisen condensation
The term “mixed condensation reaction” also applies to this reaction. One of the reagents in the cross-condensation reaction is an enolizable ester, while the other is a ketone or a non-enolizable ester. They are unable to go through enolization or self-condensation because they lack α-hydrogens.
In crossed Claisen condensation, an ester’s enolate reacts with an alternative (preferably non-enolizable) ester (an ester devoid of α-hydrogen) to produce a β-ketoester.
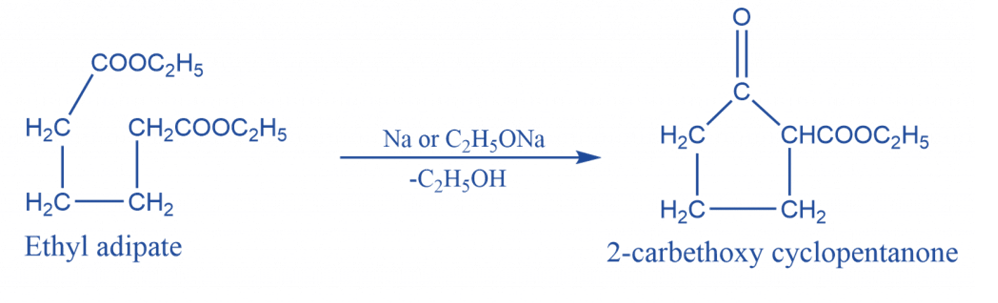
Intramolecular Claisen condensation reaction
An Intramolecular Claisen condensation is an organic process that occurs when an ester of C6, C7, or C8 dibasic acid undergoes intramolecular condensation in the presence of condensing agents such as sodium, sodium ethoxide, or potassium t-butoxide. Dieckmann condensation is another name for it.
Stobe condensation
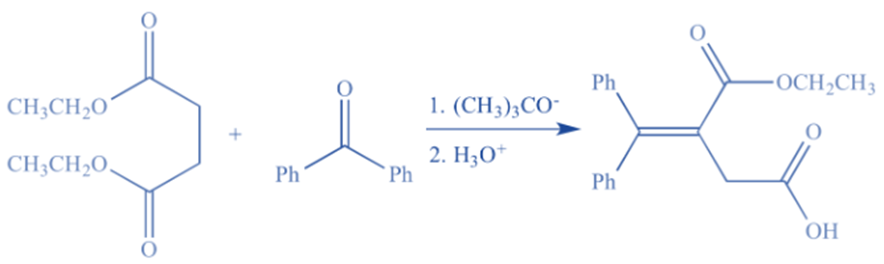
It is a condensation reaction in which an aldehyde or ketone is combined with a succinic ester in the presence of sodium or potassium ethoxide to produce unsaturated ester acid. Stobe condensation is a variant of the Claisen condensation reaction.
Applications of Claisen condensation
- It is used for the formation of the C-C framework during organic synthesis reactions.
- It is a critical method for the synthesis of β–ketoesters or β–diketones in organic chemistry and is frequently used for the synthesis of several compounds, such as medicines, flavorings, and fragrances.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry: reactions mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. roduction of polyesters, polyurethanes, and alkaline resins.
