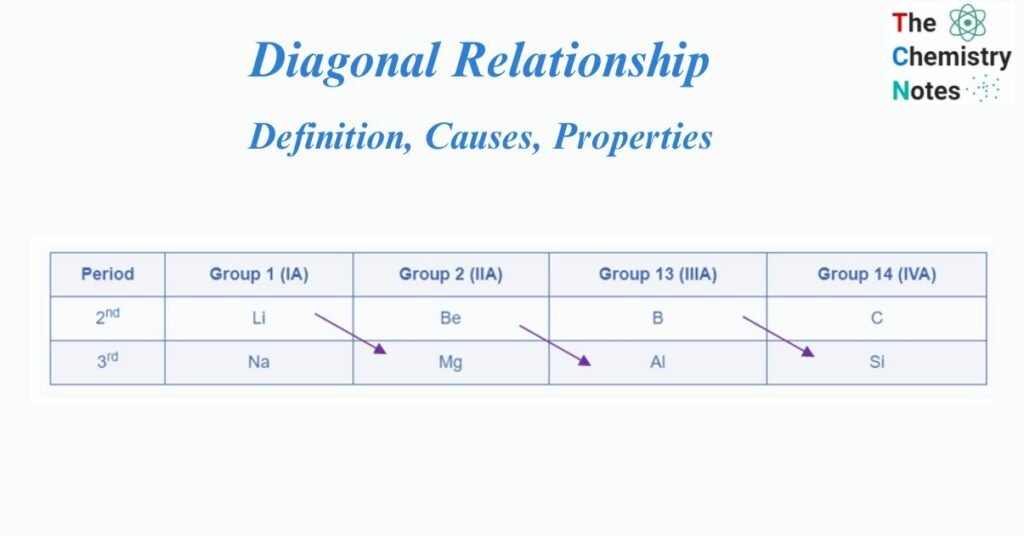
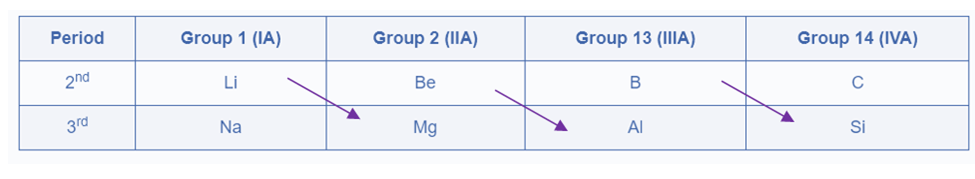
The diagonal relationship refers to the resemblance in qualities observed between two elements arranged diagonally from left to right in two adjacent periods and two adjacent groups in the periodic table.
The diagonal relationship is particularly noticeable among the lighter members of the second and third-period elements. Thus, Li (Lithium) from group IA has a diagonal – relationship with Mg (Magnesium) from group IIA, Be (Beryllium) from group IIA has a diagonal – relationship with Al (Aluminium) from group IIIA, and B (Boron) from group IIIA has a diagonal – relationship with Si (Silicon) from group IVA.
Diagonal pairs are two diagonal elements that have comparable qualities. The earlier element of the second period (Li, Be, & B) resembles the elements on the right-hand side of the third-period element (i.e. diagonally opposite element) more than another member of their group.
Cause of diagonal relationship
Diagonal relationships exist because crossing and descending the periodic table has the opposite effect. Moving through a period causes the atomic radius to decrease, whereas descending a group from top to bottom causes the atomic radius to rise. However, when traveling diagonally from one element to another, the changes cancel each other out, resulting in a nearly constant atomic radius. Electronegativity and charge density of diagonally lying components exhibit a similar connection. As a result, the diagonal relationship between elements occurs for the following reasons:
- Their similar atomic or ionic radius
- Their similar electronegativity
- Their similar charge density
These factors cause similarities in their physical and chemical properties.
Diagonal relationship between Lithium and Magnesium
Li and Mg have a diagonal relationship because of:
Both elements have nearly comparable electronegativity (Li = 1.0, Mg = 1.2).
Atomic (Li = 1.34, Mg = 1.30)
ionic radii (Li = 0.68, Mg = 0.65 ) radii of these elements are practically equal.
The physical and chemical properties of lithium that make it diagonally related to magnesium rather than vertically related to other alkali metals are given below:
- Most of the physical properties like atomic radius, ionization energy, electronegativity, polarizing power, melting point, and boiling point of Li and Mg are similar.
- Unlike the alkali metals of Group I, but like Mg in Group II, Li has greater melting and boiling points.
- Similar to lithium, Mg is also an organometallic compound. For example, RLi, RMgX
- Both Li and Mg form covalent compounds.
- Fluorides of Li and Mg form covalent compounds.
- These are active metals and decompose water to liberate H2 gas.
Li + H2O → LiOH + H2
Mg + H2O → Mg(OH)2 + H2
7. Both Li and Mg combine with oxygen to form normal oxides.
4 Li + O2 → Li2O
2 Mg + O2 → 2 MgO
8. They are good conductors of electricity.
Diagonal relationship between Beryllium and Aluminium
Be and Al has a diagonal relationship because of:
- Both elements have nearly comparable electronegativity (Be = 1.5, Al = 1.5).
- Both elements have the same covalent radius.
- Both metals have a low electropositive potential.
- Be2+ (0.033) and Al3+ (0.044) ions have about the same cationic charge-to-size ratio.
The following properties of Be and Al reflect their diagonal relationship
- Most of the physical properties like atomic radius, ionization energy, electronegativity, polarizing power, melting point, and boiling point of Be and Al are similar.
- Typically, both metals are recovered via electrolytic reduction of their oxides.
- Both metals, Be and Al, are found in nature as beryl, 3BeO.Al2O3.6SiO2.
- Be+2 and Al+3 ions have a high tendency to form complexes with ligands. Examples include [Be(H2O)4]++, [Al(H2O)6]+3, [AlF6]-3, and so on.
- Like Be, Al also forms covalent compounds.
- Oxides and hydroxides of Be and Al like BeO, Al2O3, BeOH, and Be(OH)3 are amphoteric reactions with acids and bases.
- Both metals combine with halogens to form the halides, BeX2 and Al X3. These halides are electron deficient and hence act as Lewis acids.
Diagonal relationship between Boron and Silicon
B and Si have a diagonal relationship because of:
- Both elements have nearly comparable electronegativity (B = 2.0, Si = 1.8).
- Both elements have cationic charge and cationic size ratio values that are similar.
The following points demonstrate the B and Si diagonal relationship:
- Most of the physical properties like atomic radius, ionization energy, electronegativity, polarizing power, melting point, and boiling point of Be and Al are similar.
- Because both elements are nonmetals, they are poor conductors of heat and electricity.
- The majority of compounds of these elements are covalent.
- Both elements are found in two allotropic states: amorphous and crystalline. Both elements are hard in their crystalline forms.
- None of the elements are attacked by water.
- Both silicon and boron form similar type of hydrides called silane and borane. E.g., diborane (B2H6), disilane (Si2H6).
References
- Rayner-Canham, Geoffrey (22 December 2013). Descriptive inorganic chemistry. Overton, Tina (Sixth ed.). New York
- Shriver, Duward (2006). Inorganic Chemistry (4th ed.).
- https://unacademy.com/content/neet-ug/study-material/chemistry/the-diagonal-relationship-between-group-1-and-2-elements-on-the-periodic-table/.
- https://www.embibe.com/exams/diagonal-relationship/
