In the periodic table, lithium is the first alkali. Lithium is a rare element that is mostly found in molten rock and in very small amounts in saltwater. It has the lowest melting point of any solid metal, is soft, silvery-white, reactive, and has the lightest weight. More than those of its own group, many of its physical and chemical characteristics resemble those of the alkaline earth metals.
Despite being used in numerous drug treatments because of its beneficial effects on the human brain, it is known to be non-vital in biological human processes. Humans have used lithium in thermonuclear weapons, nuclear fusion reactions, and batteries because of its reactive properties.

History
Brazilian chemist José Banifácio de Andrada found the mineral petalite in a Swedish mine in the late 1800s. The first mineral to contain Li is petalite. However, it wasn’t until 17 years later, when Swedish chemist Johan August Arfwedson examined petalite ore chemically, that the element Li was properly isolated. He noticed that this new element formed compounds similar to sodium and potassium, indicating where it would eventually fit on the periodic table.
Occurrence
Due to its reactivity, Li does not naturally occur in large quantities. The earth’s crust contains 65 parts per million (ppm) of this moderately abundant compound. The amount of Li absorbed by plants can occasionally reach around 30 ppm. Its composition in terrestrial bodies is approximately 0.1 ppm. Salt and brine deposits are two additional sources of Li . Lithium carbonate is the primary commercial form of this element. It was first found in the mineral petalite. it is also present in brine deposits and as salts in mineral springs.
Other sources of Li include pegmatite ores, such as spodumene (LiAlSi2O6) and lepidolite (of varying structure), or in amblygonite (LiAlFPO4) ores, with Li2O contents ranging between 4 and 8.5 percent.
Physical Properties of Lithium
Li is soft and silvery white and it is the least dense of the metals. It is extremely reactive and does not happen naturally. Li has a high specific heat capacity and is a liquid over a wide temperature range.
| Electronic Configuration | [He] 2s1 |
| Block, Period and Group in periodic table | s-block, Group-1, Period-2 |
| Atomic Number | 3 |
| Atomic Weight | 6.941 g.mol -1 |
| State at 20 °C | Solid |
| Melting Point | 180.50°C, 356.90°F, 453.65 K |
| Boiling Point | 1342°C, 2448°F, 1615 K |
| Density | 0.534 g/cm3 |
| Atomic radius | 152 pm |
| Vanderwaal Radius | 0.145 nm |
| Electronegativity (Pauling Scale) | 0.98 |
| Standard potential | – 3.02 V |
| Crystal Structure | body-centered cubic |
| Electron affinity (kJ mol−1) | 59.633 |
| First Ionisation energies (kJ mol−1) | 520.222 |
Chemical Properties of Lithium
Lithium’s properties are similar to those of the more common alkali metals sodium and potassium. It is therefore highly reactive with water, which it floats on, and forms potent hydroxide solutions that result in lithium hydroxide (LiOH) and hydrogen gas. It is the only alkali metal that does not form the anion, Li–, in solution or solid form.
To form compounds containing the Li+ cation, it can easily lose one of its three electrons due to its high chemical activity. Many of these have significantly different solubilities when compared to the corresponding compounds of the other alkali metals. The remarkable property of retrograde solubility, which makes lithium carbonate (Li2CO3) less soluble in hot water than in cold.
A test for lithium’s presence is based on the crimson color that Li and its compounds give to a flame. Because it reacts with the moisture in the air, it is frequently stored in mineral oil.
Reactions with Water
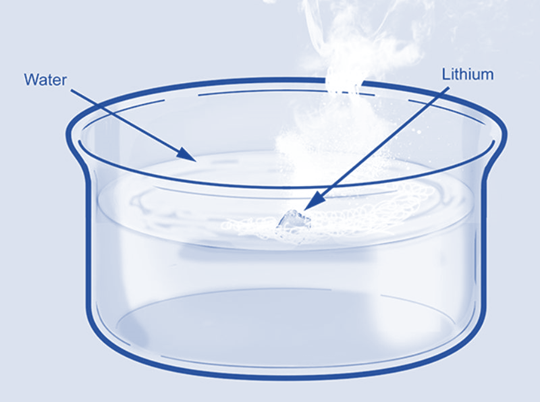
Pure Li will react with water to produce hydrogen gas and lithium hydroxide. Compared to other alkali metals like sodium and potassium, whose reactions with water are explosive, its reaction to water is less vigorous. This odd behavior is explained by its small size and high hydration energy.
2Li (s) + 2H2O (l) → 2LiOH (aq)+ H2 (g)
Reactions with Air
Due to atmospheric moisture, pure Li will produce lithium hydroxide, lithium nitride (Li3N), lithium carbonate (Li2CO3), and lithium nitride (Li3N) from N2 gas. These substances turn the silver-white metal, which usually has a white luster, black. Additionally, it will produce lithium oxide when combined with oxygen in a red flame.
4Li (s) + O2 (g) → 2Li2O
Reactivity towards Halogens
Li halides exhibit some covalent characteristics, whereas alkali metals and halogens react to form ionic halides. The high polarization capacity of Li-ion and its small size, which changes its nature to be somewhat covalent, make this exceptional. Li iodide is naturally the most covalent of Li halides.
Uses of Lithium Element
- In Ceramics: The addition of lithium carbonate during the production of ceramic bodies has many benefits, including lowering the firing temperature and reducing thermal expansion, which increases the strength of ceramic bodies and enhances their color, luster, and strength.
- In Glasses: Lithium is used in a few different kinds of glasses, including container glass, flat glass, pharmaceutical glass, specialty glass (used in touch screens), and fiberglass. Lithium increases the rate at which glass melts, which lowers viscosity and melt temperature.
- Lubricating Greases: Lithium is most frequently applicable in the production of greases. Strong base lithium hydroxide produces soap made of lithium stearate when heated in the presence of fat. Since lithium soap can thicken oils, it is used to create universal, high-temperature lubricating greases.
- In Medicine: Lithium salts are frequently applicable in medications that treat “bipolar disorder” (a mental disorder due to periods of depression, and abnormal mood that lasts from days to weeks).
- Nuclear Fuel: High-energy additives to rocket propellants include metallic Li and its hydrides, such as Li[AlH4]. In thermonuclear weapons, lithium hydride containing lithium-6 is ]works as fuel for the bomb’s fusion stage.
- In Aerospace: Aerospace companies frequently use lithium-aluminum alloys. Comparing Al-Li alloys to traditional high-strength aluminum alloys, they are lighter, and have higher tensile and yield strengths. They are resistant to the spread of fatigue cracks. It also produces other beneficial alloys, such as “white metal” bearings for metal engines made with lead and magnesium, and armor plates made with magnesium.
Lithium batteries in Electronics
Since the early 1900s, Li batteries have been used in the electronics sector. These types of batteries are non-rechargeable batteries. In the early 1980s, research on lithium-ion batteries was started, and these batteries were first commercialized in 1991. Rechargeable lithium-ion batteries can be recharged numerous times before they start to degrade. When longer battery life is essential, such as in watches, hearing aids, digital cameras, and calculators, Li batteries are used; in contrast, lithium-ion batteries are used in devices that require frequent rechargings, such as mobile phones, laptops, tablets, and emergency power backups. Li-ion batteries are also used to supply energy to medical equipment, electric vehicles, and power tools. These are applicable in portable power stations because they provide several advantages, such as high energy density, low maintenance, and high cell voltage.
Lithium for Air purification
In enclosed spaces like spacecraft and submarines, lithium salts like lithium hydroxide and lithium peroxide are used to remove carbon dioxide and thereby purify the air. Due to its low weight compared to other alkaline hydroxides, lithium hydroxide is preferred. It absorbs carbon dioxide from the atmosphere by forming lithium carbonate.
2LiOH (s) + CO2 (g) → Li2CO3 (s) + H2O (g)
Lithium peroxide (Li2O2) in presence of moisture, reacts with carbon dioxide to form lithium carbonate and oxygen.
2 Li2O2 + 2 CO2 → 2 Li2CO3 + O2
Health and Environmental Effects of Lithium
Health Effects
- Fire or explosion can result from a variety of reactions that emits noxious or irritating gases (or fumes) during a fire.
- When combustible materials and water are in contact, there is a risk of fire and explosion. Burning sensation on inhalation, Cough, Laboured breathing, respiration difficulty, painful throat.
- A red color skin, Skin scalds, Pain, Blisters, Reddened eyes, and incredibly deep burns.
- If ingested: Pain in the abdomen, abdominal discomfort burning feeling Nausea, shock or pass out, Vomiting and Weakness.
- Eyes, skin, and respiratory tracts are all negatively impacted by the substance. corrosive when consumed. Lung oedema from the substance inhalation is possible. The signs of lung oedema frequently do not appear for several hours, and physical activity makes them worse.
Environmental Effects
Air in the presence of nitrogen, oxygen, and water vapor will react with metallic lithium. As a result, a coating of lithium hydroxide (LiOH), lithium carbonate (Li2CO3), and lithium nitride forms on the surface of the lithium (Li3N). Due to its extreme corrosiveness, lithium hydroxide poses a potentially serious risk. Water organisms should receive special consideration.
Animals exposed to the effects would primarily experience tissue irritation. If significant amounts of this solution are released, the main impact on plants would be the modification of the salinity of contaminated soils.
Toxicity, Safety, and Precautions
Lithium is reactive and a dangerous explosion hazard. It is corrosive when in contact with moisture or water.
Reproductive Toxicity: Rat offspring NOEL >90 mg/kg; maternal NOEL 30 mg/kg. 2-generation study ongoing.
Carcinogenicity Status: Li2CO3 is not listed as a carcinogen or suspected carcinogen by IARC, NTP, OSHA, or ACGIH.
Irritancy of Product: Li2CO3 moderately to severely irritates the eyes and may irritate the skin.
Safety
Eye Contact: Quickly brush off the excess chemical from the face. Immediately flush with large amounts of water for at least 60 minutes, lifting upper and lower lids. Remove contact lenses, if worn, while flushing. Seek medical attention immediately.
Skin Contact: Quickly remove contaminated clothing. Immediately blot or brush off excess chemicals and wash gently with large amounts of water for at least 60 minutes. Seek medical attention immediately.
Inhalation: Remove the person from exposure. Begin rescue breathing (using universal precautions) if breathing has stopped and CPR if heart action has stopped. Medical observation is recommended for 24 to 48 hours after overexposure, as pulmonary edema may be delayed.
EMERGENCY NUMBERS
Poison Control: 1-800-222-1222
CHEMTREC: 1-800-424-9300
NJDEP Hotline: 1-877-927-6337
National Response Center: 1-800-424-8802
Precautions
- Inform and train employees about potential hazards.
- Keep an eye on chemical concentrations in the air. If concentrations are higher than the recommended exposure levels, use engineering controls.
- Give out emergency showers and eyewash stations. If skin comes into contact with a dangerous substance, wash or take a shower. Always wash after a shift at work.
- If clothing gets contaminated, change clothes immediately.
- To wash contaminated clothing, receive specialized training.
- In locations where chemicals are handled, processed, or stored, avoid eating, smoking, and drinking.
- Before consuming anything, smoking, drinking, applying cosmetics, or using the restroom, wash your hands thoroughly.
- Wear eye protection with side shields or goggles. Wear a face shield along with goggles when working with corrosive, highly irritating, or toxic substances.
Also go through the post https://scienceinfo.com/sulfur-element/
Interesting Facts About Lithium
- Because it is metal, it is so soft that a knife can be used to cut it.
- Lithium has such a low density that it floats on water.
- Lithium reacts with water, making it difficult to extinguish a fire; a powder extinguisher is needed.
- Burning it produces a red, bright flame.
- Since it is the lightest metal, it can be alloyed with other metals like copper and aluminum to create strong lightweight metal.
- Along with hydrogen and helium, lithium was one of the three elements that a big bang produced in significant amounts.
- The element with the lowest density and greatest weight is lithium.
Find out more interesting facts on the video
References
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN0-8493-0464-4
- Kipouros, Georges J and Sadoway, Donald R. “Toward New Technologies for the Production of Lithium.” Journal of Metals. The Minerals, Metals, & Materials Society. Volume 50, No. 5 May 1998: 24-32.
- https://studiousguy.com/lithium-uses/
- https://melscience.com/US-en/articles/chemical-and-physical-characteristics-lithium-and-/
- https://www.chemicool.com/elements/lithium.html
- https://byjus.com/chemistry/lithium/
- https://www.britannica.com/science/lithium-chemical-element#ref278859
- https://www.lenntech.com/periodic/elements/li.htm
- Shin, Y. J. ; Kim, I. S. ; Oh, S. C. ; Park, C. K. and Lee, C. S. “Lithium Recovery from Radioactive Molten Salt Wastes by Electrolysis.” Journal of Radioanalytical and Nuclear Chemistry. Akadémiai Kiadó and Springer Science+Business Media B.V. Volume 243, No. 3 March 2000: 639-643.
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_sBlock_Elements/Group__1%3A_The_Alkali_Metals/Z003_Chemistry_of_Lithium_(Z3)

