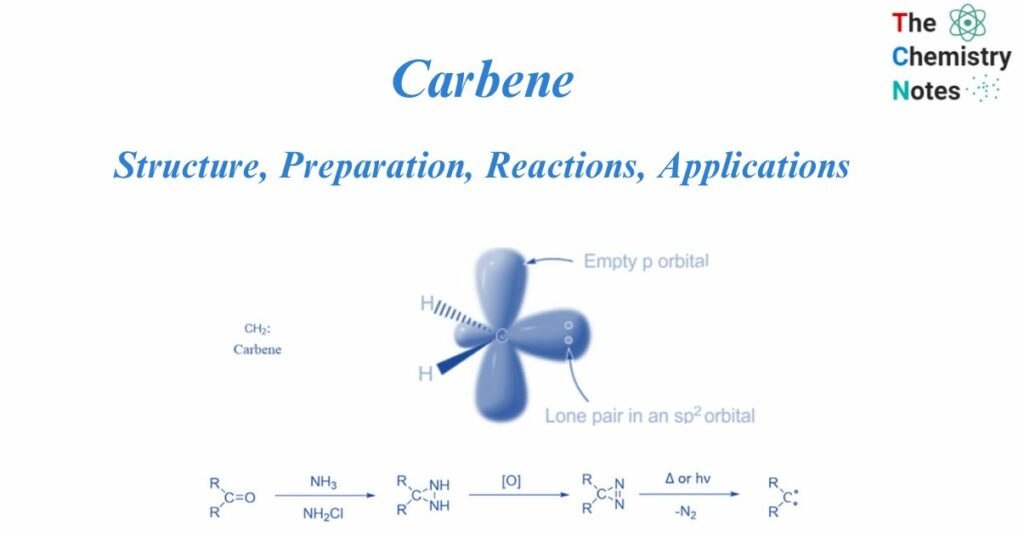
A carbene is a molecule that has a neutral carbon atom with a valence of two and two unshared valence electrons. They are neutral transitory carbon intermediates. They have two valence electrons that are not shared, as well as a neutral carbon atom with a valency of two. They are denoted as R-(C:)-R’, or R2C: R denotes the substituent group or hydrogen atoms. They can be singlets or triplets depending on their structure.
Carbenes are uncharged, electron-deficient chemical entities that consist of a divalent carbon atom surrounded by a sextet of electrons and two substituents.
The simplest carbene is the CH2 compound, which is known as methylene and was initially used in the nineteenth century. Dumas first described his attempts to prepare the parent carbene (CH2) through the dehydration of methanol in 1835.
What are carbenes?
They are chemical intermediates that have a short lifetime. They are extremely reactive but neutral due to electron deficiency. The type of the substituent group linked to the carbon atom also influences the compound’s chemical reactivity and electron structure. They exhibit electrophilicity due to the presence of only six electrons in their outer shell, the strength of which rises with the presence of an electron-withdrawing group linked to the carbene. However, when powerful donor groups are connected to the carbene, it functions as a nucleophile.
Nomenclature of carbene
They are divalent carbon compounds that are usually referred to together with substituents. Substituents are listed first, followed by the term carbene.
CH3CH: – Ethylene
CH2: – Methylene
These are termed with the suffix -yilidene if the divalent carbon atom is in a ring or the carbene electrons are on a carbon-carbon double-bond carbon atom.
H2C=C: – Vinylidene
Structure of carbene
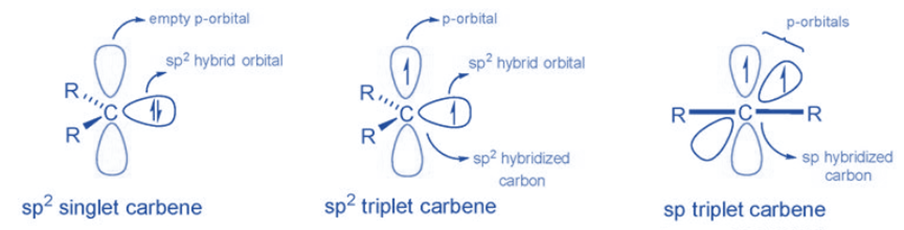
Two bonding electrons (both in sp2-orbitals) and two non-bonding electrons are found in carbenes. There are two types: singlet carbenes and triplet carbenes, depending on whether the non-bonding electrons are in the same or distinct orbitals. According to the valence bond theory, carbenes often include sp2 hybridized carbon atoms. Two of the three sp2-hybrid orbitals form covalent connections with their carbene substituents, leaving two unoccupied orbitals consisting of the sp2-hybrid orbital and the p orbital. These vacant orbitals must be filled with two non-bonding carbene electrons.
When two electrons are placed in the same orbital, the carbene is known as a singlet carbene since the electron spins are in different directions. According to Hund’s law, when electrons are positioned in various orbitals, parallel spin is favored, and the resulting carbene is known as triplet carbene.
Singlet and triplet carbene
The two unshared electrons in singlet carbene exist as a spin pair. As a result, the overall spin of a singlet carbene is zero. According to valence bond theory, the molecule forms a sp2 hybrid structure. It has a bond angle of 102° and is commonly found in aqueous media. The singlet carbene is paramagnetic because it contains an electron pair.
Depending on the hybridization, the triplet carbene has a linear or curved structure. The linear structure of the sp hybrid carbene is different from the bending structure of the sp2 hybrid carbene. Except for those with nitrogen, oxygen, or sulfur atoms, as well as halides directly bonded to divalent carbon, the majority of carbenes have a non-linear triplet ground state. In this carbene, electrons have parallel spin, resulting in a total spin of one. In a gaseous form, triplet carbenes are more stable. The triplet carbene is diamagnetic because it includes unpaired electrons.
In general, the singlet state is more stable than the triplet state in most organic molecules. As a result, these molecules’ ground states are singlet. The triplet state exists only as an excited or high energy level in certain compounds. The nature of the substituents influences the electronic characteristics of carbenes. If the carbene carbon’s substituents contain electron-withdrawing groups, the carbene favors the singlet form. Electron withdrawing groups stabilize the σ-orbital connected to the carbene carbon inductively, increasing the energy difference between the σ- and π-orbitals. As a result, electrons enter the σ-orbital, leaving the π-orbital unoccupied. If the substituents connected to the carbene carbon via the σ-bond are electron-donating groups, the carbene favors the triplet form.
Furthermore, atoms connected to the carbene carbon with non-bonding electron pairs (nitrogen, oxygen, sulfur, halogen, etc.) easily give their electrons to the carbene’s unoccupied p-orbital. Thus, the resonance structure of the -donor atoms stabilizes the singlet state, and carbenes prefer the singlet configuration in the ground state.
Preparation of carbene
From 𝝰-elimination reaction
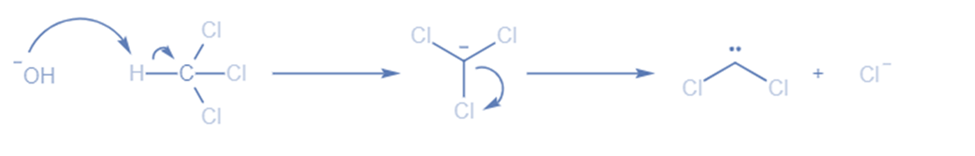
𝝰-elimination reactions occur when both the proton and the leaving group are present on the same carbon atom. A strong basic, such as NaOH, is used in this method to remove an acidic proton located close to the electron-drawing group, resulting in the creation of carbanion.
They can be formed via the reaction of aqueous potassium hydroxide (KOH) with chloroform. The base extracts the proton from the chloroform in the first step, resulting in the generation of an anion. The anion loses the chloride ion in the second stage, yielding the divalent carbene with two unshared pairs of electrons.
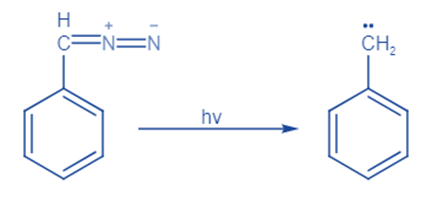
From diazo compound
Diazo compounds (RR’C=N2) are an important precursor. By removing nitrogen gas via heating, diazo compounds with 1,3-dipolar structures, such as diazomethane, can be transformed into their related carbenes. Different metal complexes can serve as catalysts in this process.
By removing nitrogen from the diazo compounds, carbenes are formed. Heat, light, and copper catalyze this process.
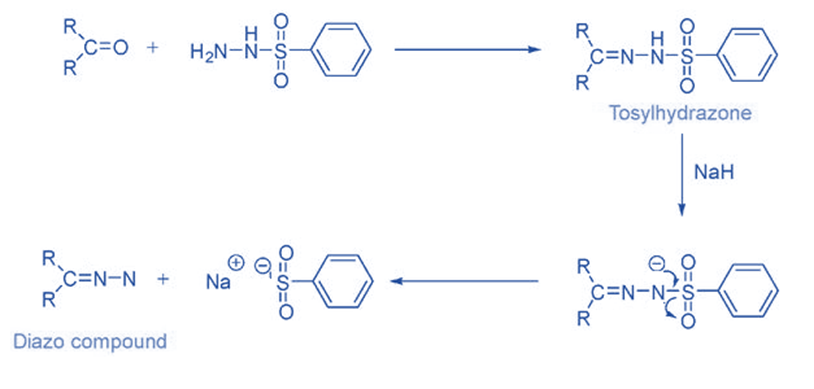
From tosyl hydrazone
Ketones and aldehydes easily react with hydrazine to form hydrazone compounds, which are then oxidized with metal salts such as Ag2O, HgO, MnO2, and Pb(OAc)4 to produce the diazo compound. Diazo compound is unstable or dangerous to use, it is usually preferable to use a diazo precursor. Hydrazones are the easiest and most popular compounds used for this.
Tosylhydrazones, which are formed by reacting aldehydes and ketones with p-toluene sulphonyl hydrazide, are the most well-known and commonly utilized carbene precursors.
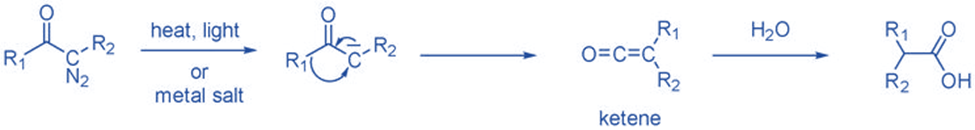
From ketene
Ketenes can create carbene by removing CO molecules during thermolysis or photolysis. Ketenes are not commonly employed since they are not a readily available precursor and polymerize under reaction conditions. Ketene has been widely utilized to produce CH2.
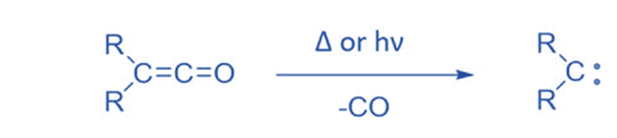
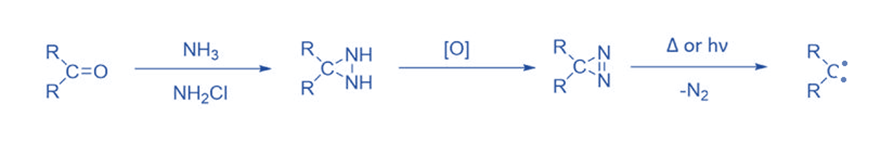
From three-membered ring compounds
Three-membered rings with a high ground state energy due to steric strain frequently break down to give carbene intermediates when heated or irradiated. Diazirines are the most important carbene precursors of the three-membered ring. Diazirines, the cyclic isomers of diazoalkanes, similarly break down to yield carbenes when exposed to heat and light. Diazirines are synthesized from ketones by reacting them with ammonia and chloramine, then oxidizing the resultant diaziridine. This approach is commonly used, particularly for halocarbenes.
From sterically hindered alkene
If the alkene is very sterically inhibited, the π-bond is weakened due to significantly reduced p-p overlap and planarity distortion. As a result, the ground state energy increases, allowing for dissociation to carbenes via heating.

Reactions of Carbene
As they are a highly reactive intermediate, they interact in a variety of ways. Depending on the reaction circumstances, they can undergo insertion, addition, and rearrangement reactions.
Cycloaddition reaction
They often react electrophilically, they produce cyclopropane compounds via a [2 + 2] cycloaddition process with double bonds. Since 1954, this technique, the most common reaction of carbene intermediates, has been widely employed as a synthetic route to cyclopropane.
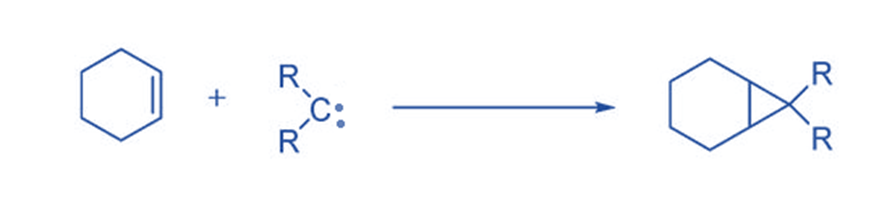
Insertion reaction
They undergo a specific process to become stable by inserting between C-H or C-C bonds. The electronic structure of carbene is crucial in insertion reactions. Singlet carbenes typically enter into bonds in a single step with configuration retention. With triplet carbenes, the situation is different. Because triplet carbenes are radicals, they first extract hydrogen from the C-H bond and combine it with two new radicals. A new C-C bond is created when these radicals are combined. In the meantime, as the radical configuration isomerizes, a racemic mixture is generated as a result of the reaction. Tertiary carbon-hydrogen bonds are favored for the insertion of triplet carbenes because they create stable radicals.
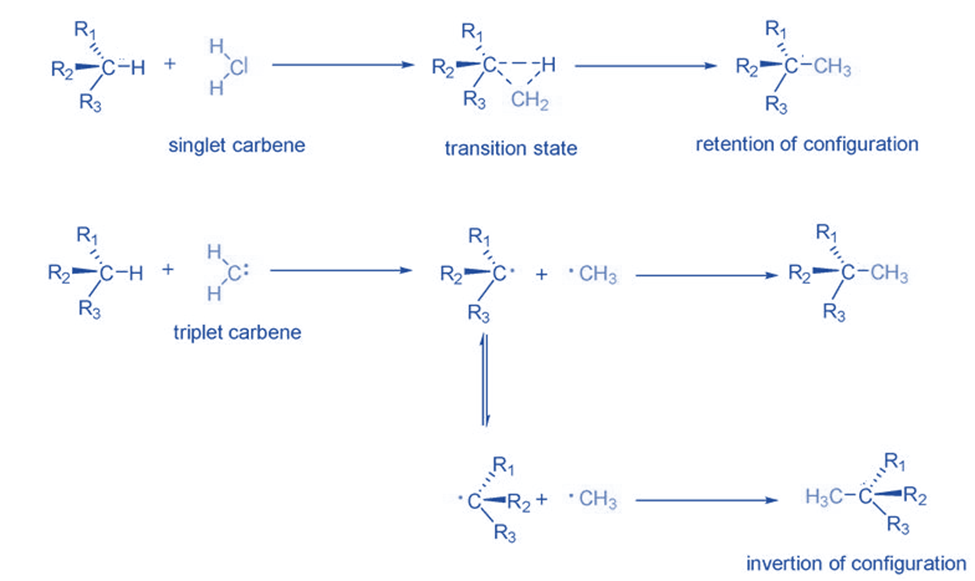
Rearrangement reaction
They are intermediates that lack an electron and have an empty p-orbital, so when an atom or group of atoms from a neighboring carbon migrates to the electron-deficient core, it undergoes a simple rearrangement and simultaneously creates a new C=C bond. Because the migration capacity is H>> aryl> alkyl, this rearrangement is commonly referred to as a 1,2-shift and usually involves the migration of a hydrogen atom. This hydrogen shift refers to the intramolecular addition of the carbene to the adjacent C-H bond.
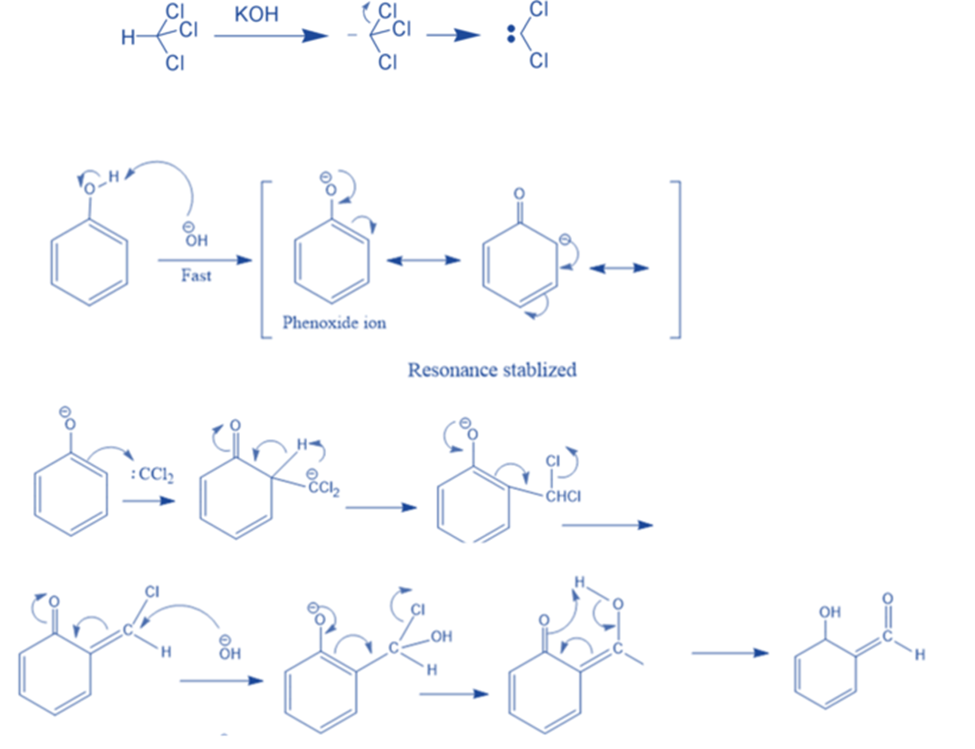
Reimer Tiemann Reaction
When phenol is heated in the presence of chloroform and alkali, salicylaldehyde is formed. This is known as the Reimer-Tiemann reaction. In this reaction, first, a strongly basic hydroxide solution is used to deprotonate the chloroform. The chloroform carbanion is formed by removing the hydrogen atom. The chloroform carbanion will swiftly undergo alpha elimination, yielding dichlorocarbene(CCL2), the reaction’s major reactive species. Then, extracts the hydrogen atom from the -OH group to generate the phenoxide ion. The phenoxide’s negative charge is delocalized into the aromatic ring, making it more nucleophilic. The addition of dichlorocarbene results in the formation of an intermediate i.e., dichloromethyl-substituted phenol. The resulting intermediate is then exposed to basic hydrolysis to yield ortho-hydroxybenzaldehyde.
Applications of carbene
Their main application is as highly efficient organocatalysts and transition metal catalysts in the synthesis of complex chemicals. As a result, they play an important role in the field of organometallic chemistry. It is widely utilized in the industrial manufacturing of tetrafluoroethylene (TFE), a precursor to Teflon. The intermediate produced in this reaction is difluorocarbene.
