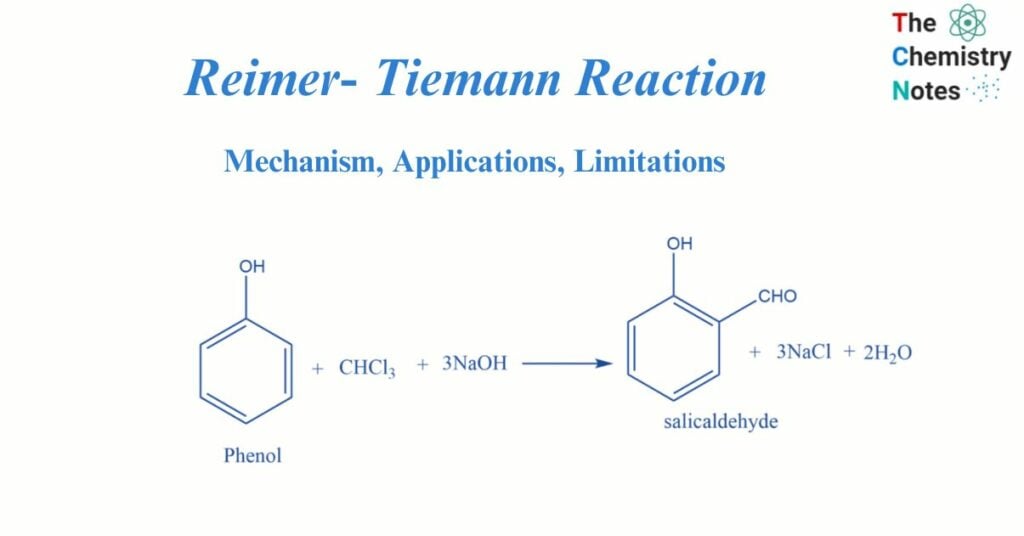
The Reimer- Tiemann reaction, named after scientists Karl Reimer and Ferdinand Tiemann, is a type of substitution reaction. The reaction is utilized for the ortho-formylation of C6H5OH (phenols). One of the most basic instances of this reaction is the transformation of phenol to salicylaldehyde.
It is an electrophilic substitution reaction in which phenol (or other electron-rich aromatic compounds like naphthols, pyrroles, indoles, and so on) is warmed with chloroform and aqueous alkali to produce ortho or para-formylated product.
This reaction produces a mixture of ortho and para isomers, with the ortho isomer being the predominant result. The para-isomer is the main product if any of the ortho positions of the benzene ring is occupied. The two isomers are separated via fractional distillation, which involves distilling unreacted phenol and the ortho-isomer over the para-isomer. Ortho products are significant for two reasons:
1. The probability factor
2. H-bonding in the final salicylaldehyde
Interesting Science Videos
What is Reimer- Tiemann Reaction?
When phenol is heated in the presence of chloroform and alkali, salicylaldehyde is formed. This is known as the Reimer-Tiemann reaction.
A biphasic solvent is needed to complete the reaction since the hydroxides found in chloroform are not easily soluble. This biphasic system is primarily composed of an aqueous hydroxide solution with an organic phase containing chloroform. The separated reagents are combined so that the reaction can take place. Several strategies are employed to bring these reagents together. Some examples include quick mixing, phase transfer catalysts, and the application of an emulsifying agent.
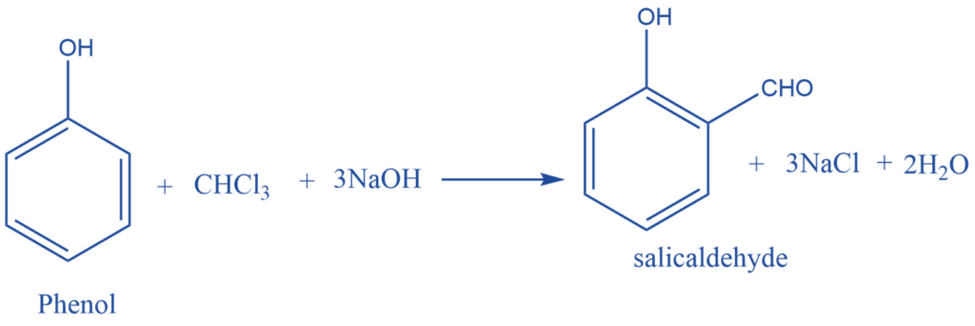
Mechanism of Reimer- Tiemann Reaction
Step I: First, a strongly basic hydroxide solution is used to deprotonate the chloroform. Deprotonation is the elimination of the hydrogen atom. The chloroform carbanion is formed by removing the hydrogen atom. The chloroform carbanion will swiftly undergo alpha elimination, yielding dichlorocarbene(CCL2), the reaction’s major reactive species.
Step II: Base extracts the hydrogen atom from the -OH group to generate the phenoxide ion. The phenoxide’s negative charge is delocalized into the aromatic ring, making it more nucleophilic.
Step III: The addition of dichlorocarbene results in the formation of an intermediate i.e., dichloromethyl-substituted phenol. The resulting intermediate is then exposed to basic hydrolysis to yield ortho-hydroxybenzaldehyde.
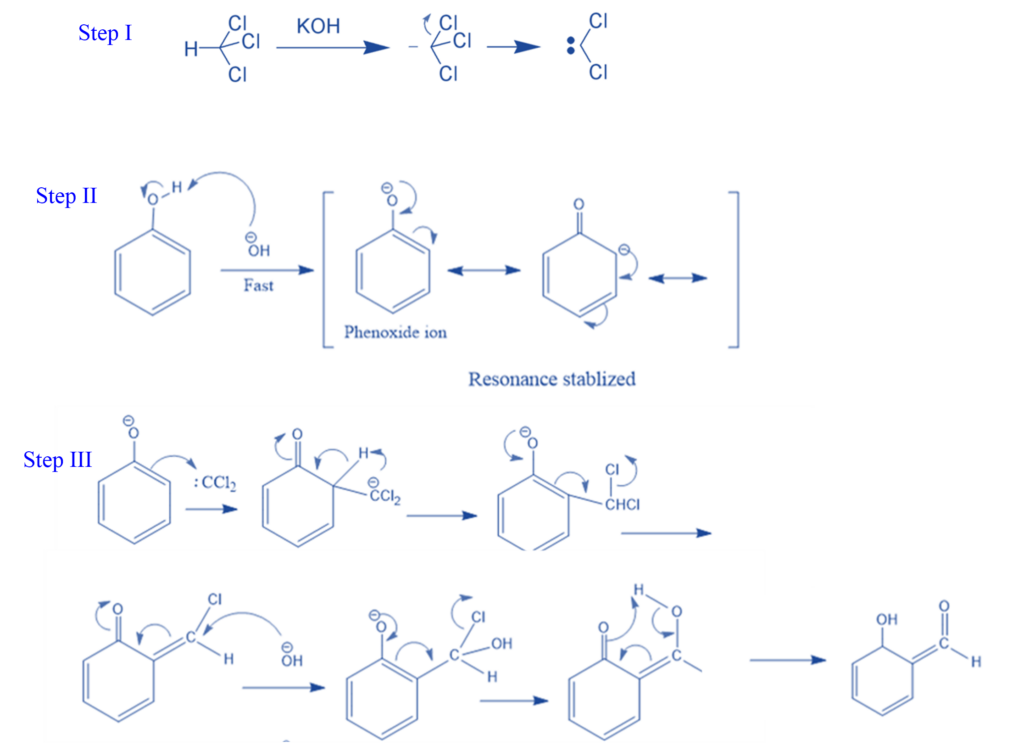
Abnormal Reimer- Tiemann Reaction
Certain compounds, such as pyrrole, indene, and indole, react with chloroform and bases (such as NaOH, KOH, and others) to produce a rearrangement product rather than the Riemer Tiemann product. The term “abnormal Reimer Tiemann reaction” refers to this unusual kind of reaction.
For example, Pyrrole is transformed to 3-chloropyridine when heated with a mixture of chloroform (CHCl3) and alkaline solution (NaOH). Dichlorocarbene is generated when pyrrole is treated with a strong base such as NaOH or NaOMe, KOH. The dichlorocarbene is electrophilically coupled with pyrrole to generate the unstable dichlorocyclopropane, which subsequently rearranges to a 3-chloropyridine.
Conditions of Reimer- Tiemann Reaction
- The Reimer-Tiemann reaction must be performed in a biphasic solvent solution. A biphasic mixture is a combination of immiscible stages that often contain a natural solvent and an aqueous phase.
- By using 1,4-Dioxane, an emulsifying agent, phase transfer catalysts, or rapid-fire mixing, the two reagents are combined.
- This reaction performs well when other hydroxy-aromatic chemicals, such as naphthols, are employed.
- Heat is required to initiate the chemical process.
- Once the reaction starts, it becomes quite exothermic.
Applications of Reimer- Tiemann Reaction
- By replacing the chloroform with carbon tetrachloride, Reimer-Tiemann reactions can generate phenolic acids such as 2-hydroxybenzoic acid.
- The Reimer- Tiemann reaction is primarily used in the ortho formulation of phenols.
- Since the Reimer-Tiemann reaction is the only approach that doesn’t require acidic or anhydrous conditions, direct formylation of aromatic compounds is frequently carried out safely and is the simplest way for formylation.
- The synthesis of 3-halo pyridines, naphthalenes, quinolines, etc. can be accomplished using it.
- It can be used to make Salicyaldehyde, which is used in perfumes, as a flavoring ingredient, and for medical purposes.
Limitations of Reimer- Tiemann Reaction
The following are the limitations of the Reimer-Tiemann Reaction:
- yields of aromatic aldehydes are low.
- The two-phase reaction system prevents a sufficient mass transfer between the two layers.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
