Fullerene is a carbon allotrope whose molecules are carbon atoms joined by single and double bonds. As a result, a closed or partially closed cage-like structure (a mesh of fused rings) containing atoms is formed. The fullerene molecule in this form can be a hollow sphere, an ellipsoid, or a tube, or it can have a variety of other shapes and sizes. Carbon nanotubes are tube-like structures formed when carbon molecules are organized in a cylindrical configuration.
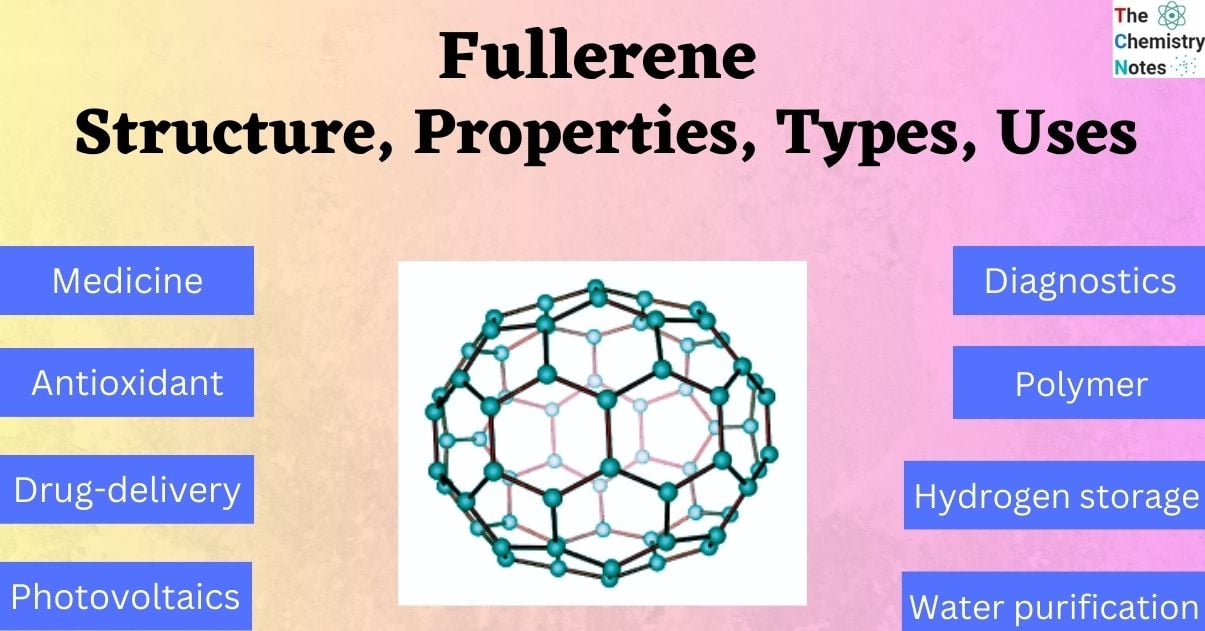
It is produced by applying heat to graphite in an electric arc with an inert gas. The molecules generated by the thick carbon dioxide are mostly C60 fullerene molecules. Its shape is like a soccer ball, and it is commonly known as Buckminsterfullerene.
What are Fullerenes?
A fullerene is a carbon allotrope. Fullerenes are carbon molecules with spherical (buckyballs), ellipsoid, tubular (nanotubes), or a combination shape (nanobuds). They are made up of hexagonal and pentagonal (occasionally also heptagonal) rings, with the latter being required for the molecule’s curvature.
Fullerenes have numerous applications. They can be used to reinforce fibers in textiles. Fullerenes are also employed in cosmetics products and have a variety of medical applications.
They have a structure comparable to carbon nanotubes, but have higher concentrations and are less hollow. The C60 molecule is the most prevalent type of fullerene. It is shaped like a truncated icosahedron or a soccer ball. Each vertex has one carbon atom, and each carbon atom is connected to another via two single bonds and one double bond.
Discovery of Fullerene
- Sir Harold W. Kroto of the United Kingdom and Richard E. Smalley and Robert F. Curl, Jr. of the United States discovered fullerene in 1985. R.F. Curl, H.W. Kroto, and R.E. Smalley are awarded the Nobel Prize in Chemistry by the Royal Swedish Academy of Sciences in 1996 for their discovery of fullerenes.
- Krätschmer and Huffmann discover a method for producing C60 and other fullerenes in large numbers in 1990.
- K3C60, an alkali intercalated substance, was discovered to be superconducting at 18K in 1991, setting a record for organic superconductors.
- AC60 is first prepared on January 1, 1992. These phases have an air-stable polymer structure.
Structure Of Fullerene
Fullerenes are highly symmetrical, particularly the C60 sphere. Fullerenes have a structure similar to graphite; they are a sheet of connected hexagonal rings that feature pentagonal (or occasionally heptagonal) rings that prevent the sheet from being flat. Buckyballs or buckytubes are names given to them based on their shape. Nanotubes are cylinders of fullerene. With no two pentagons sharing an edge, C60 is the most common fullerene.
Fullerenes, such as C70, C76, C82, and C84, exhibit ellipsoidal or deformed spherical structures, and fullerene-like assemblies up to C240 have been discovered. A remarkable property of these structures is that the area within the carbon cage can house atoms, ions, or tiny molecules. Endohedral fullerenes are a type of fullerene. Although the cavity of C60 is small, encapsulated helium, lithium, and atomic nitrogen compounds have been found. Huge fullerenes are known to encapsulate lanthanide metals.
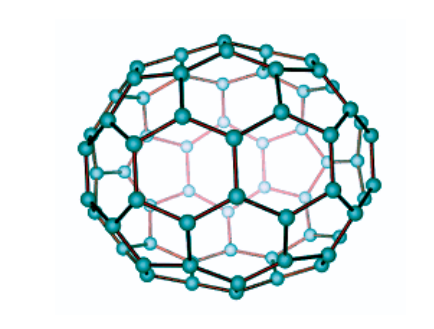
- Buckminsterfullerene C60 is a 60-Carbon atom Fullerene compound. The atoms form hexagonal and pentagonal rings. A spherical structure is formed from 20 hexagonal rings and 12 pentagonal rings.
- Each Carbon atom in a fullerene molecule forms three covalent connections. Single and double covalent bonds exist between carbon atoms. Double bonds are known as pi-bonds (π), and the electrons that are shared in a π bond are known as π electrons. These electrons combine to produce an electron cloud that wraps around the entire structure of the Buckyball molecule. The molecule simply takes up area that can hold this electron cloud.
- The C60 molecule, with this electron cloud, has a diameter of 1.0 nm. This is also known as the fullerene Van Der Waals diameter.
- The C60 molecule has a diameter of 0.7 nm measured from nucleus to nucleus (excluding the electron area).
Types Of Fullerene
Fullerenes are classified into two primary families based on their unique features and applications. Closed buckyballs and open-ended cylindrical carbon nanotubes are among the two families. There are, however, hybrid structures that can exist between these two types. Carbon nanotubes, which are nanotubes bound with hemispherical meshes, or huge “Bucky buds” are two possibilities.
Some of the fullerene types are discussed below:
Buckminsterfullerene
Buckminsterfullerene has pentagonal and hexagonal rings and is the smallest fullerene molecule. Buckminsterfullerene has the empirical formula C60, which indicates the 60 carbon atoms in each molecule. It can occur naturally and be found in soot.
Buckminsterfullerene has a truncated icosahedron structure, similar to football, which has twenty hexagons and twelve pentagons. A carbon atom is present in each polygon and bonds along each polygon edge. The molecule’s Van Der Waals diameter is around 1.1 nanometers (nm), and the spacing between nuclei in the molecule is 0.71 nm.
Buckytubes
Buckytubes are a type of carbon nanotube. These fullerenes are essentially unsaturated dodecahedra. These are also some of the tiniest fullerene members. It has the structural formula C20. They can, for example, be a few micrometers to millimeters in length and a few nanometers in width. They have structures that are both closed and open-ended. Buckytubes (carbon nanotubes) offer a wide range of applications due to their macroscopic properties.
Carbon Nanotube
This fullerene features cylindrical or hollow tubes with very small diameters, usually a few nanometers broad. They can, however, range in length from micrometers to many millimeters. When it comes to carbon nanotube qualities, they can be both closed and open-ended. They have unique macroscopic properties as a result of their unique molecular structure. High tensile strength and electrical conductivity, high ductility, heat conductivity, and relative chemical inactivity are a few of them. The megatube is another kind that we come upon. These are slightly larger in diameter than nanotubes and typically have walls of varying thicknesses.
Physical Properties Of Fullerene
Fullerenes have a peculiar structure with great strength and endurance, thermal and electrical conductivity, and corrosion resistance. They are also unstable and are unaffected by many solvents or gases. Some of the physical properties of fullerene are included here:
- When the temperature is changed, fullerene’s behavior and structure change. At higher temperatures, the fullerene is transformed into the C70 form.
- Fullerenes are insoluble in water and most other solvents.
- Fullerene has weak intermolecular forces. To overcome these forces, less energy is required. They are smooth and slick, with a low melting point.
- At room temperature, fullerenes do not evaporate. They are highly flammable.
- Fullerenes are unable to conduct electricity. In their purest form, they are insulators. They only function as superconductors or semiconductors when doped with alkali metals.
- Under changing pressures, the structure of fullerenes can vary.
- Fullerene’s ionization enthalpy is 7.61 electron volts.
- Fullerene has an electron affinity of 2.6 to 2.8 electron volts.
- The sublimation temperature is 600oC. This means that at 600oC, it directly transitions from its solid form to its gas state, without first entering the liquid stage.
- They are capable of displaying the photochromic effect. In a nutshell, it is a shift in light transmission based on intensity.
- Fullerenes are relatively harmless and inert, but they can produce active derivatives.
| Chemical formula | C60 |
| Molar mass | 720.66 g·mol−1 |
| Appearance | Dark needle-like crystals |
| Density | 1.65 g/cm3 |
| Melting point | sublimates at ~ 600 °C (1,112 °F; 873 K) |
| Solubility in water | insoluble in water |
Chemical Properties Of Fullerene
Some of the chemical properties of fullerene are included here:
- Fullerenes are not soluble in water, but they dissolve readily in organic solvents. Fullerenes can be dissolved by solvents such as chlorobenzene, 1,2,3-trichloropropane, and toluene.
- Fullerene C60 functions as a catalyst and speeds up the oxidation of hydrogen sulphide, H2S, to create sulfur (S).
- Because fullerenes are chemically reactive, they can be added to polymer structures to produce new co-polymers with precise physical and mechanical properties.
- Fullerenes are potent antioxidants that react quickly and efficiently with free radicals, which are frequently the cause of cell damage or death. They act like “radical sponges,” absorbing and neutralizing 20 or more free radicals per fullerene molecule.
- C60 and higher fullerenes are hydrophobic, meaning they have a low solubility in water and cannot form stable hydrates. Polar group functionalization can be used to increase the hydrophilic affinity and consequently the solubility of fullerenes in water.
Synthesis Of Fullerene
Fullerenes were first synthesized by laser vaporization of carbon in an inert atmosphere, although the amount of fullerene produced was quite little. However, considerable amounts of fullerene C60 were later produced through graphite arc heating and laser irradiation of poly aromatic hydrocarbons (PAHs).
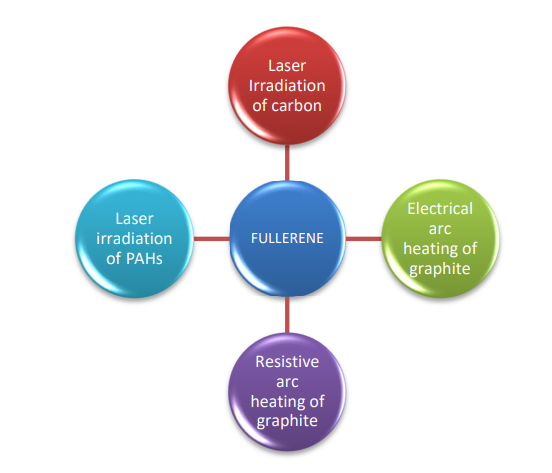
Laser irradiation of carbon
A pulsed laser focused on graphite targets in an inert atmosphere (helium) produces fullerenes in a supersonic expansion nozzle. A focused pulsed laser is used to vaporize carbon from a revolving solid disc of graphite into a high-density helium flow.
Electric arc heating of carbon
Kratchmer and Huttman developed this approach in 1990. An electric arc is created between graphite rods in an inert atmosphere, resulting in a fluffy condensate (Soot).
below. Toluene extractible fullerenes are present in a portion of this fluffy condensate. The fullerenes in the soot are then extracted by solvation in a small amount of toluene. After extraction, the toluene (solvent) is removed using a rotary evaporator, and the solid mixture, consisting primarily of C60 with a trace of higher fullerenes, is subjected to a liquid chromatography process to obtain pure C60.
Resistive arc heating of graphite
This method involves resistive heating of carbon rods in a partial helium atmosphere; the resistive heating of carbon rods causes the rod to emit a faint grey white plume, soot-like substance made up of fullerenes, which is collected on glass shields surrounding the carbon rods.
Laser irradiation of PAHs
Direct synthesis of fullerenes has been developed as a method of obtaining novel Homologues of the fullerenes family that would otherwise be difficult to obtain in sufficient quantities through the uncontrolled process of graphite evaporation.This method of fullerene synthesis is based on polycyclic aromatic hydrocarbons (PAHs) with pre-existing carbon frameworks. Under flash vacuum pyrolysis (FVP) conditions, such PAH molecules are “rolled up” to form fullerenes. It has been reported that when a polycyclic aromatic hydrocarbon with 60 carbon atoms is laser irradiated at 337mn wavelength, it forms fullerene C60.
Uses/Applications of Fullerene
The unique physical and chemical properties of fullerenes have prompted many researchers to investigate the use of this molecule and its functionalized derivatives in a variety of fields, including medicine, photovoltaics, gas adsorption/storage, and pharmaceuticals, to name a few.
Use in Medicine
Size, hydrophobicity, electrical configuration, and three-dimensional ability are some of the most appealing properties of fullerene, propelling them to the forefront of medical chemistry. Despite evident solubility issues, fullerenes stand out as possible therapeutic agents due to their excellent carbon cage structure and broad possibilities for functionalization.
Antioxidants and biopharmaceuticals
Fullerenes react readily with free radicals because of the presence of large amounts of conjugated double bonds and low lying Lowest Unoccupied Molecular Orbitals (LUMO), it has been reported that about 34 methyl radicals have been added onto a single C60 molecules as such it is considered the world’s most efficient radical Scavenger
Antimicrobial/Antibacterial Activity
Hydrophilic fullerene derivatives, such as fullerols and amino fullerene, have demonstrated antibacterial activity and, as a result, have piqued the interest of researchers for potential use in water treatment systems. Attachment of numerous, hydroxyl, carboxylic acid, and glycolic oxide on C60 has also been demonstrated to induce photodynamic cytotoxicity against pathogenic microbes, including multi-antibiotic-resistant bacteria ). The ability of C60 to form reactive oxygen species (ROS) such as singlet oxygen and superoxide through photosensitization when it interacts with organic solvents has been attributed to its biocidal action.
Antiviral Potency
Fullerene derivatives’ antiviral properties have been attributed to their unparalleled molecular cage structure and antioxidant properties. According to research, fullerene derivatives can inhibit and form a complex with HIV protease. Fulleropyrrolidine with two amino groups have also been demonstrated to be efficacious against HIV – I and HIV – 2.
Diagnostics
Endo Fullerene is created when a metal ion is placed into a fullerene cage, and this endohedral metallo-fullerene cage could function as an isolation chamber that separates reactive atoms from the biological environment. The gadolinium-encapsulated endohedral metallofullerene (EMFs) have been announced as top prospects for future-generation magnetic resonance imaging (MRI) contrast agents. Biodistribution studies have also revealed the localization of metallofullerenes to macrophages, implying that these species are selectively targeted to microphage-rich tissues and may be very valuable chemotherapeutic agents for the treatment of bone cancer and leukemia.
Drug Delivery
Because of their superior biocompatibility, selective targeted distribution, and controlled release of pharmaceuticals, fullerenes can be converted into suitable drug carriers for cellular delivery. Fullerenes become water soluble by attaching hydrophilic species to them, making them exceptional in drug and gene cellular delivery. Because functionalized fullerenes can pass cell membranes and connect to mitochondria, they can be programmed to release medications slowly in order to get the best possible results. Investigations have revealed that DNA-functionalized fullerenes outperform commercially available lipid-based vectors in terms of efficacy.
Photovoltaics
Fullerenes have been employed as important components in artificial photosynthetic processes due to their excellent electron-accepting capacity and low reorganization energies, which facilitate electron transfer processes.
Metalloporphyrins, cyanines, phthalocyanines, Ruthenium bipyridine complexes, boron-dipyrrin, metallocenes, and tetrathiafulvalene are examples of organic and transition metal containing electron donating units that have been linked to fullerene cages. Consequently fullerenes have found usefulness in photoelectric devices, electrochemical materials, doping impurity for conductive polymer films, gas sensors and superconductors
Polymeric Materials Based on Fullerene
The excellent electrical characteristics of fullerene-based polymeric materials have led to their production and subsequent application in a variety of fields. Fullerene-based polymeric materials can be made in a variety of ways, including direct attachment of fullerene materials to polystyrene in friedel-crafts reactions or indirect linkage of fullerenes C60. Fullerene C60 reacts with either of these spacer groups to form insoluble PtC60. These redox-active fullerene-based polymers can also be generated or manufactured as polymer films on an electrode when C60O is reduced
Water Purification
Despite the fact that the most abundant fullerene (C60) has a well-known photocatalytic capability, its limited solubility in water is a major drawback for water purification and environmental applications. The ability of fullerenes to act as adsorbents for organic molecules has been explored and employed in the speciation study of various metal forms. The efficient generation of reactive oxygen species (ROS) by photosynthetic fullerene derivatives (Amino Fullerene and fullerols) promises to pave the way for the development of C60 -fullerene mediated oxidation processes, which might be very useful for environmental applications.
Storage of Hydrogen
One distinguishing feature of fullerenes that makes them ideal hydrogen storage molecules is the hydrogenation of their C-C bonds to form C-H bonds, which have lower bond energies; therefore, heating breaks the C-H and returns the molecule to its original distinctive fullerene structure. Because of their chemistry and cage molecular structure, thus-fullerenes can retain up to 6.1% of hydrogen
Energy Materials
Super-capacitor surface electrodes are typically formed of a conductive layer of carbon, and their capacitance is primarily determined by surface area, pore size distribution, electrolyte accessibility, and electrical conductivity. However, limited electrolyte accessibility to solid surfaces usually leads to low capacitance, hence nanotechnology has opened up new avenues through the use of a diverse variety of carbon-based nano materials, all in an attempt to overcome the aforementioned deficiencies.
Frequently Asked Questions (FAQ)
Can fullerene conduct electricity?
Yes, fullerenes are good conductor of electricity.
Explain the Structure of Fullerene
Fullerenes have a structure similar to graphite, which is made up of hexagonal sheets of carbon molecules. Fullerenes, unlike graphite, are often pentagonal in shape and so do not lie in flat sheets. The most common fullerenes are made up of sixty carbon atoms (C60) that are held together by single and double bonds.
Who discovered fullerene?
In 1996, Robert Curl, Harold Kroto, and Richard Smalley won the Nobel Prize in Chemistry for the discovery of the fullerenes.
Video on Fullerene
References
- https://mstnano.com/properties-of-fullerenes/
- https://byjus.com/jee/fullerene/
- Bakry, R., Vallant, R. M., Najam-ul-Haq, M., Rainer, M., Szabo, Z., Huck, C. W., & Bonn, G. K. (2007). Medicinal applications of fullerenes. International journal of nanomedicine, 2(4), 639.
- Yadav, J. (2018). Fullerene: properties, synthesis and application. Research & Reviews: Journal of Physics, 6(3), 1-6.
- Tsachouridis S and Papaioannidou P (2010). Fullerenes: Chemical structure and properties. Front. Pharmacol. Conference Abstract: 8th Southeast European Congress on Xenobiotic Metabolism and Toxicity – XEMET 2010. doi: 10.3389/conf.fphar.2010.60.00206
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/fullerenes/
- https://collegedunia.com/exams/fullerene-chemistry-articleid-5459
- https://pubchem.ncbi.nlm.nih.gov/compound/Buckminsterfullerene#section=Physical-Description
- Gharbi, N., Pressac, M., Hadchouel, M., Szwarc, H., Wilson, S. R., & Moussa, F. (2005). [60] Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Letters, 5(12), 2578-2585.
- https://unacademy.com/content/jee/study-material/chemistry/fullerenes/
- Physical Properties of Organic Fullerene Cocrystals, Roberto Macovez* Volume 4 – 2017 https://doi.org/10.3389/fmats.2017.00046
- https://pdfs.semanticscholar.org/3cc1/0a179a47bed8f86e1a8f4a748aac59022bd0.pdf

