One of the most important topics in general chemistry is stoichiometry. Stoichiometry is a fundamental concept in chemistry that allows us to calculate the amounts of reactants and products using balanced chemical equations. In general, all reactions are determined by one main factor: the amount of substance present. As a result, it could be introduced as “Mass Relations.”
What is Stoichiometry?
Stoichiometry is a term derived from the Greek words stoicheion (meaning “element”) and metron (meaning “measure”). The study of quantitative relationships in substances and their reactions is known as stoichiometry. Stoichiometry calculations frequently deal with the mass or volume of products and reactants.
A chemical equation that is balanced conveys a lot of information in a clear and concise manner. Chemical formulas identify the reactants and products involved in the chemical change, allowing the reaction to be classified. The relative numbers of these chemical species are provided by coefficients, allowing for a quantitative assessment of the relationships between the amounts of substances consumed and produced by the reaction.
Pronunciation
Pronounce stoichiometry as “stoy-kee-ah-met-tree” or abbreviate it as “stoyk.”
Laws of Stoichiometry
The laws of stoichiometry are those that deal with the composition of a substance by mass or volume.
The laws of stoichiometry are:
- Law of conservation of mass
- Law of definite/constant proportion
- Law of multiple proportion
- Law of reciprocal proportions
- Law of gaseous volume
Law of Conservation of mass
Proposed by: French chemist Antoine Lavoisier (1774)
Verified By: Landolt
It states, “mass is neither gained nor lost during a chemical reaction” or that “in a chemical reaction, the total mass of reactants consumed equals the total mass of products formed.”
It is also known as law of indestructibility of matter.
If reactants A and B combine to form products C and D, then the sum of the masses of A and B equals the sum of the masses of C and D.
Exceptions: Nuclear Reaction, Radioactive Disintegration
Limitation: Not entirely applicable for nuclear reactions in which the mass of the products formed is slightly less than the mass of the reactants due to the conversion of some mass into energy according to the equation E=mc2.
Law of definite/constant proportions
Proposed By: French chemist Joseph Louis Proust in 1799
Verified By: Stats and Richards
It states, “The same chemical compound always contains the same elements combined together in a definite proportion by mass, regardless of the compound’s origin or mode of formation.”
Carbon and oxygen atoms are combined in the ratio 3:8 by mass in carbon dioxide gas obtained either by heating limestone or by combustion of carbon or hydrocarbon.
Exception: Isotopic reaction, non-stoichiometric compounds (Berthollide compounds) e.g. Wustite: Fe0.94O or (VOx), x can be any number between 0.6 and 1.3.
Limitations: This law does not apply if the element involved in compound formation has multiple isotopes. In contrast to the first case, CO2 formed by the combination of C-14 isotope and oxygen contains carbon and oxygen atoms in the ratio of 7:16 by mass. Also, this law does not apply to non-stoichiometric compounds such as wustite, where the iron-oxygen ratio ranges from 0.83:1 to 0.95:1.
Law of multiple proportions
Proposed By: John Dalton in 1803
Verified By: Berzelius
It states, “When two or more different elements combine to form two or more chemical compounds, the mass of one of the elements that combines with a fixed mass of the other bears a simple whole number ratio.”
Nitrogen, for example, combines with oxygen to form a series of nitrogen oxides, with the ratio of masses of oxygen that combine with 28 parts by mass of nitrogen being 1:2:3:4:5, demonstrating the law of multiple proportion.
Law of reciprocal proportions
Propose By: Richter in 1792
Verified By: Stas
It states, “The ratio of the masses of two elements which combine separately with a fixed mass of the third element is either same or some simple multiple of the ratio of the masses in which these elements combine with each other”
Consider the elements hydrogen, oxygen, and sulfur as an example. Separately, oxygen and hydrogen react with sulfur to form SO2 and H2S. They also combine to form H2O, as shown in the figure.
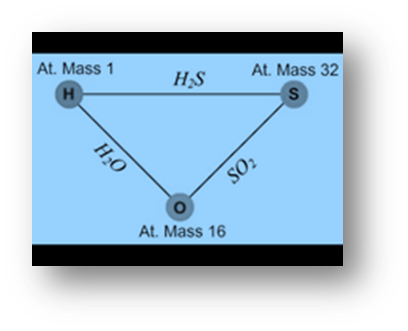
Illustration:
32 parts by mass of sulfur combine with 32 parts by mass of oxygen to form sulfur dioxide (SO2).
2 parts hydrogen by mass combine with 32 parts sulphur by mass to form hydrogen sulphide (H2S).
As a result, the ratio of hydrogen and oxygen masses that combine with fixed mass, i.e. 32 parts by mass of sulphur, is
2:32 i.e. 1:16…………….. (i)
In water (H2O), two parts hydrogen combine with 16 parts oxygen. The mass ratio of hydrogen to oxygen is
2:16 i.e. 1:8…………….. (ii)
These two ratios are related as 1/16:1/8, which is a simple multiple ratio of 1:2.
Law of gaseous volume
Proposed By: Gay Lussac in 1808
It states, “when gases react together under similar conditions of temperature and pressure, they do so in volumes that bear a simple ratio to one another and the volumes of the products as well if they are also gases.”
For example, if steam is formed by combining hydrogen and oxygen gas, there is a simple volume ratio.
2H2 (g) + O2 → 2H2O (g)
The volume ratio of reactants and products is 2:1:2.
Stoichiometric coefficient
The stoichiometric coefficient, also known as the stoichiometric number, is the number of molecules involved in the reaction. Any balanced reaction has an equal number of components on both sides of the equation, as you can see if you take a closer look.
Stoichiometric coefficients can be either fractions or whole numbers. The coefficients essentially assist us in determining the mole ratio of reactants to products.
We can deduce from:
2Na (s) + 2HCl (aq) → 2NaCl (aq) + H2 (g)
2 moles of HCl will react with 2 moles of Na(s) to form 2 moles of NaCl(aq) and 1 mole of H2(g). If we know how many moles of Na reacted, we can use the ratio of 2 moles of NaCl to 2 moles of Na to calculate how many moles of NaCl were produced, or we can convert to NaCl using the ratio of 1 mole of H2 to 2 moles of Na. This is referred to as the coefficient factor. The balanced equation allows you to convert information about one reactant or products change into quantitative data about another reactant or product. Understanding this is critical in order to solve stoichiometric problems.
Limiting Reagent
The reactant that is completely consumed during a reaction is known as the limiting reagent, and it controls when the reaction comes to an end.
The exact amount of reactant required to react with another element can be calculated using reaction stoichiometry. If the reactants are not mixed in the correct stoichiometric proportions (as indicated by the balanced chemical equation), one will be completely consumed while the other will be left over. The limiting reagent is completely consumed; it prevents the reaction from proceeding because there is none left to react with the excess reactant.
Guidelines for Reaction Stoichiometry
- Make a balanced equation.
- Determine the number of moles of the species whose mass is given.
- Using the equations coefficients, convert the moles of the given substance into moles of the desired substance.
- Determine the weight of the desired species.
References
- Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten
- https://byjus.com/jee/stoichiometry-and-stoichiometric-calculations/
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Chemical_Reactions/Stoichiometry_and_Balancing_Reactions
- https://openstax.org/books/chemistry-2e/pages/4-3-reaction-stoichiometry
