mCPBA (Meta-chloroperoxybenzoic acid ) is a peroxycarboxylic acid with the chemical formula C7O3H5Cl. It is a white crystalline powder with a melting point of 90 degrees Celsius. mCPBA is a powerful oxidising agent that is commonly employed as an oxidant in chemical synthesis. It may catch fire if it comes into contact with a combustible material.
What is mCPBA?
Meta-chloroperbenzoic acid (mCPBA) is a peroxycarboxylic acid that is commonly employed as an oxidant in organic synthesis due to its diverse oxidizing potency and ease of handling.
Its reactivity is distinguished by a weak O-O bond and a nucleophilic OH group. mCPBA’s O-O bond transfers an oxygen atom to electron-rich substrates, while its nucleophilic attack on ketones and aldehydes results in the insertion of an oxygen atom.
It is soluble in CH2Cl2, CHCl3, 1,2-dichloroethane, ethylacetate, benzene, and ether. However, m-CPBA is moderately soluble in hexane and completely insoluble in water. It is easy to handle, combustible, and hygroscopic. Pure mCPBA, on the other hand, is shock-sensitive and can deflagrate. However, 85% mCPBA is not shock-insensitive and needs to be stored in a refrigerator in tightly sealed containers. mCPBA irritates mucous membranes, the respiratory tract, the eyes, and the skin, and skin contact with mCPBA results in burns and blisters.
mCPBA is a versatile peracid that can be used in laboratories. The m-CPBA is utilized as an oxidizing agent in various oxidative transformations including Baeyer-Villiger oxidation, production of epoxides, oxaziridines, α–disulfines, sulfoxides and sulfones, N-oxides, and ketones. The ease of handling meta-chloroperbenzoic acid is due to its presence as a powder (crystalline white solid) that can be stored in the refrigerator. Nonetheless, a material with a purity greater than 75% is rarely commercially available since the pure compound is not particularly stable. mCPBA is a potent oxidizing agent that, when in contact with flammable material, can produce a fire.
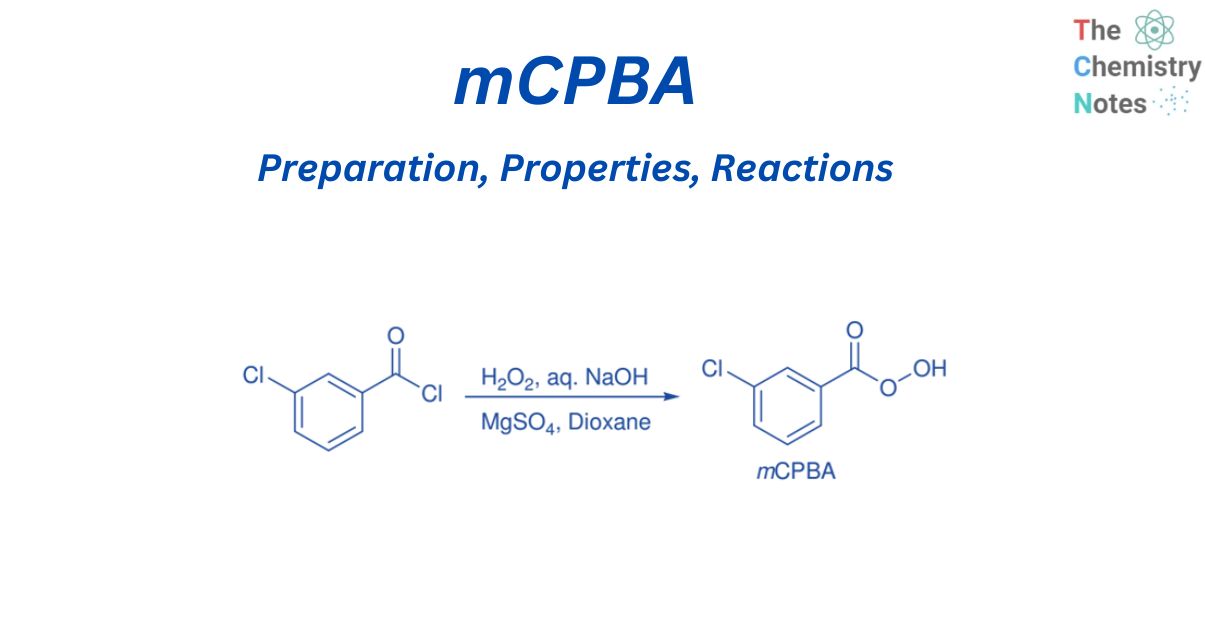
Preparation of mCPBA
mCPBA can be synthesized by reacting m-chlorobenzoyl chloride with H2O2 in the presence of MgSO4.7H2O, aqueous NaOH, and dioxane
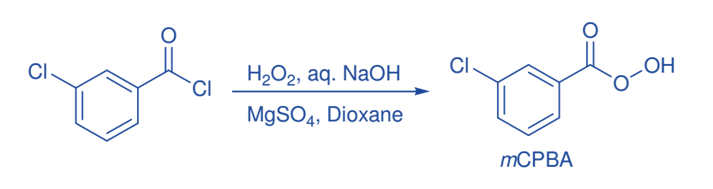
Some important reactions of mCPBA
Baeyer-Villiger oxidation
The Baeyer-Villiger oxidation is a crucial step in the production of lactones and esters from ketones. The reaction is critical for the production of lactones. The nucleophilic attachment of the peroxide reagent to the carbonyl carbon of the substrate yields the tetrahedral intermediate in the Baeyer-Villiger oxidation by peroxy acid. The intermediate undergoes intramolecular rearrangement of an alkyl or aryl substituent from the central carbon to the nearby oxygen, with the breaking of the weaker O-O bond and simultaneous creation of the ester (or lactone) and a carboxylic acid.
Baeyer-Villiger oxidation is regioselective when the two ligands on the carbonyl carbon in the ketone are different. During this reaction, the highly substituted alpha carbon migrates more preferentially.
The reaction’s regiospecificity is determined by the relative migratory ability of the substituents linked to either side of the carbonyl group. Substitutes that can stabilize the positive charge migrate easily. The following is the migratory aptitude of different substituents:
H > 3o-alkyl > cyclohexyl > 2o– alkyl > benzyl > aryl > 1o – alkyl > methyl.
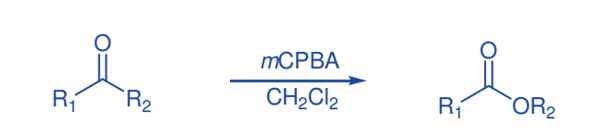
Meisenheimer rearrangement
The Meisenheimer rearrangement permits both functionality and stereochemical information to be transferred. The oxidation of a variety of aziridines with mCPBA produced compounds derived from a [2,3] Meisenheimer rearrangement of the original N-oxide, followed by additional oxidation to produce nitrones.
It appears that the reaction proceeds by oxidizing the aziridines to produce N-oxide, which then undergoes quick Meisenheimer rearrangement, followed by additional oxidation of the nucleophilic nitrogen, and finally base catalyzed N-O bond cleavage to produce nitrone.
Rubottom oxidation
The synthesis of -hydroxy ketones is accomplished through the reaction of silyl enol ethers with mCPBA, followed by rearrangement. Typically, silyl enol ethers are treated with a small excess of m-CPBA in CH2Cl2 at 0 oC, followed by workup with pentane. Rubottom oxidation products show that the hydroxy group is introduced regiospecifically and that no exchange happens with respect to the position of the initial carbony1 group in the ketonic precursor. Nonaqueous workup of the oxidation products, on the other hand, produced trimethylsiloxy ketones.
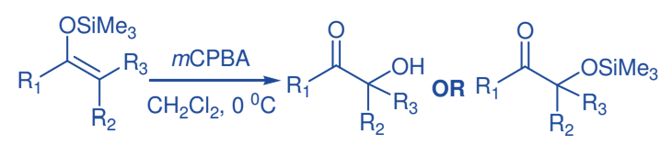
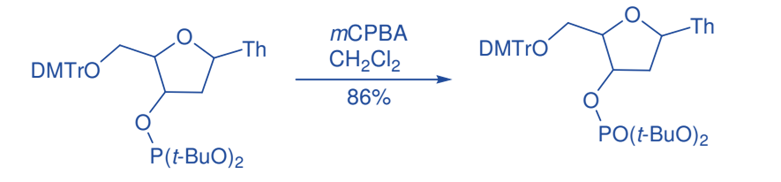
Oxidation of Phosphorous
Phosphate was stereospecifically oxidized by m-CPBA, yielding phosphate in 86% yield. Similarly, mCPBA oxidation of thiophosphate triesters produced phosphate esters with configuration retention.
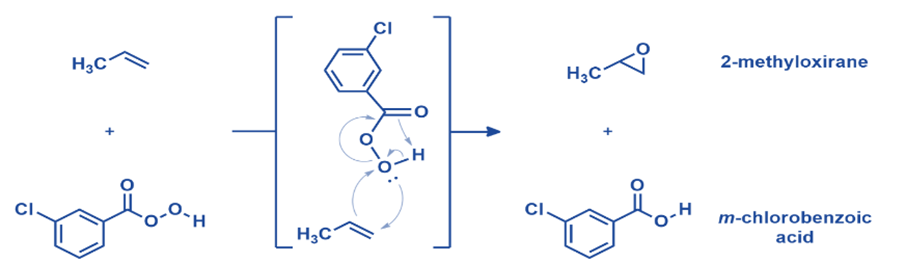
Epoxidation of olefin
On a laboratory scale, mCPBA remains one of the most useful synthetic techniques for alkene epoxidation. Electron-donating substituents on the carbon-carbon double bond and electron-drawing groups on the peroxy acid enable oxygen atom transfer from mCPBA to an alkene. A wide range of substituted olefins produced high yields. The mechanism of mCPBA’s reaction with alkenes is complicated. Two additional C-O bonds are created as the alkene approaches the peroxy acid. Simultaneously, the O-O connection is broken, causing the carbonyl to attack the hydrogen, releasing its electrons to the oxygen. Five separate bonds are made and broken in one concerted stage, making it an appealing mechanism. A single chemical step usually involves the movement of no more than three electron pairs.
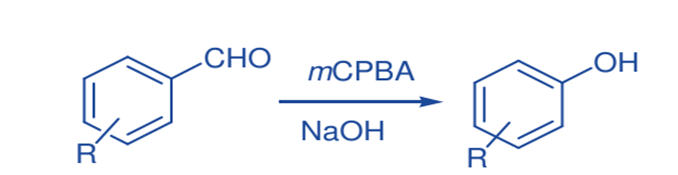
Dakin Oxidation
Solid-state Dakin oxidation with m-CPBA and several nonactivated benzaldehydes yielded phenols with a notable reduction in reaction time and yield.
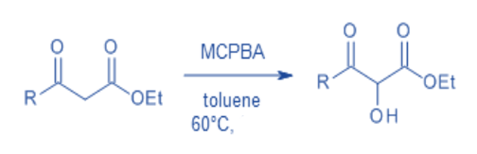
Hydroxylation of β-oxo esters and β-oxoamides
Under mild reaction conditions, a direct metal-free α-hydroxylation of α -unsubstituted β-oxoesters and β-oxoamides using m-chloroperbenzoic acid as the oxidant offers simple metal-free access to key α-hydroxy-β-dicarbonyl moieties. Additionally, the hydroxylated products are easily transformed into vicinal tricarbonyl compounds, which are excellent synthetic precursors.
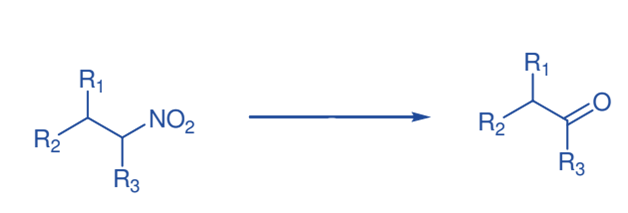
Nef reaction
TiCl4 and mCPBA were used to convert nitro compounds to carbonyl compounds. With the Nef reaction, various structurally varied secondary nitro compounds have thus been transformed into carbonyl
compounds.
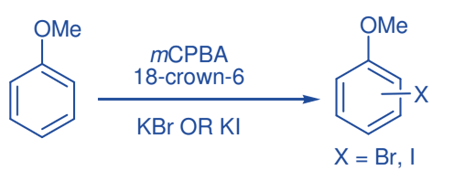
Halogenation of phenyl ether
Several types of phenyl ethers could be mono-brominated in the aryl ring in good yields with potassium bromide in the presence of 18-crown-6 upon oxidation with mCPBA. Similarly, monoiodination of phenyl ethers and free phenols was achieved by employing potassium iodide in the presence of 18-crown-6 upon oxidation with mCPBA.
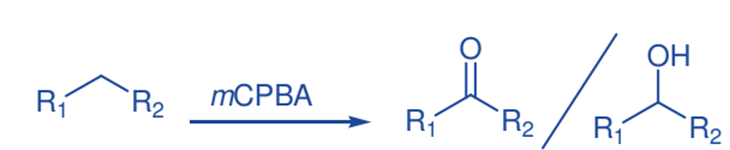
Oxidation of C-H bond
m-CPBA catalyzes the hydroxylation of aliphatic hydrocarbons in the presence of electron-deficient iron(III) porphyrin complexes. High yields of alcohol products and small amounts of ketones were produced. The hydroxylation of cis- and trans-1,2-dimethylcyclohexane produced extremely stereospecific tertiary alcohols, showing that these reactions are very stereospecific.
Selective functionalization of saturated hydrocarbons is critical in synthetic organic chemistry, and great effort has been expended in recent decades to develop efficient techniques for both regio- and stereoselective C-H bond activation.
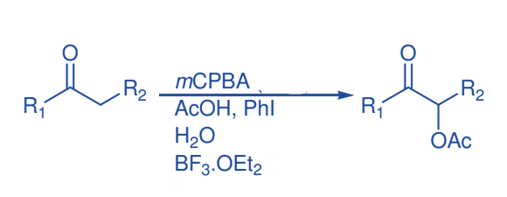
α-Acetoxylation of ketones
At room temperature, the reaction of acetophenone with dried mCPBA in acetic acid in the presence of a catalytic quantity (10 mol%) of iodobenzene, BF3.Et2O and water produce α- acetoxyacetophenone . The presence of water is critical to the effectiveness of acetophenone α–acetoxylation since α–oxidation was almost completely prevented in the absence of water, with 95% ketone recovery.
Safety Concern
- This product stimulates the skin and may cause inflammation. Wear gloves, clothes, and safety eyewear.
- Under normal temperature and pressure, this product is stable. Keep flammable products away from the storage area. Stay away from heat, sparks, burning, collisions, and friction.
References
- https://www.chemeurope.com/en/encyclopedia/Meta-Chloroperoxybenzoic_acid.html.
- https://link.springer.com/article/10.1007/s10562-010-0460-7
- https://chemistryscore.com/mcpba-reagent/
- https://www.organic-chemistry.org/chemicals/oxidations/meta-chloroperbenzoicacid.shtm.
- https://www.masterorganicchemistry.com/2011/06/17/reagent-friday-m-cpba-meta-chloroperoxybenzoic-acid/
