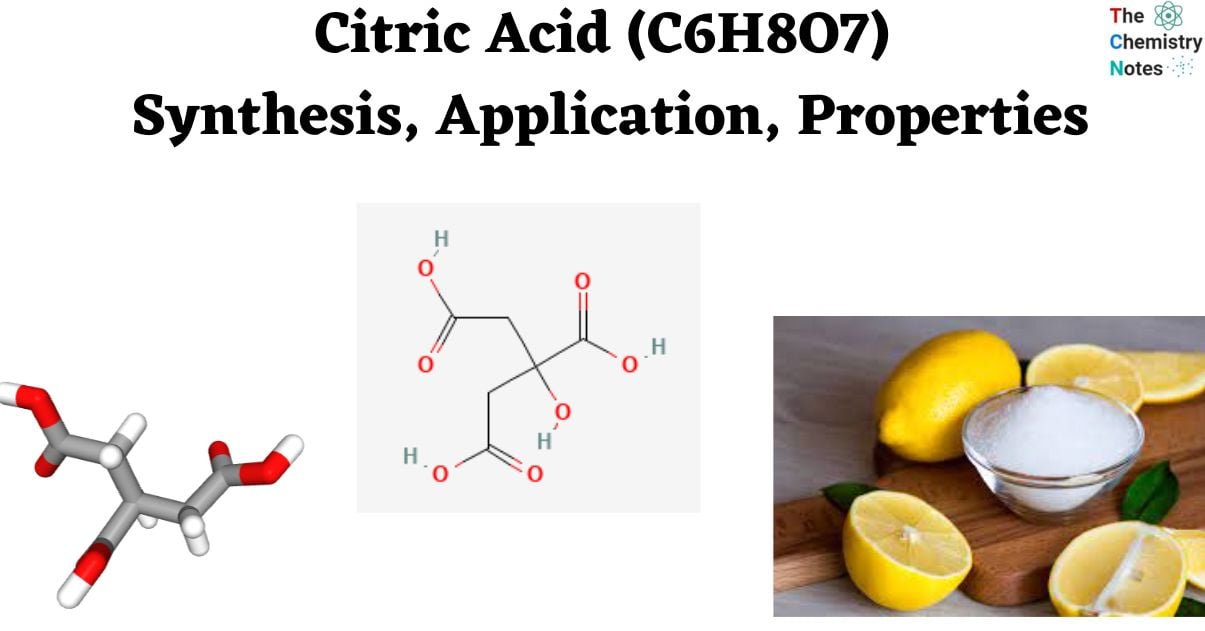
Citric acid is classified as a weak organic acid with the chemical formula C6H8O7. It is commonly found in the form of a white, crystalline powder. This substance serves as a natural food preservative and is commonly utilized to impart an acidic or sour flavor to various food products and beverages. In the field of biochemistry, the compound plays a crucial role as an intermediate in the Krebs (citric acid) cycle, making it a fundamental component in the metabolic processes of nearly all organisms.
Citric acid is a naturally occurring acid that can be found in various citrus fruits, tomatoes, and a wide range of other fruits and vegetables. However, it is worth noting that the presence of citric acid is particularly discernible in citrus fruits such as lemons and limes, which exhibit a significant concentration of this compound. It may come as a surprise, but citric acid can constitute up to 8% of the fruit’s overall dry weight.
This compound serves as an intermediary in the citric acid cycle within the field of biochemistry and is commonly observed in the metabolic processes of all aerobic organisms. The utilization of citric acid is widely recognized for its numerous benefits. Citric acid is commonly utilized in various consumer goods as well as the food and beverage industries. Citric acid is an acidulant primarily manufactured through fermentation and citrate, and it is a derivative of citric acid. Citric acid finds applications across multiple industries. Citric acid has the potential to serve as an environmentally friendly cleaning agent.
Interesting Science Videos
History of Citric Acid
- The initial discovery of Citric acid occurred in 1784, courtesy of chemist Carl Wilhelm Scheele. Scheele conducted experiments involving the crystallization of Citric acid derived from lemon juice.
- In 1917, James Currie, an American food chemist, identified Aspergillus niger, a mold capable of producing citric acid through the metabolic breakdown of sucrose or glucose.
- This discovery indicated that this technology outperformed standard citrus fruit extraction in terms of efficiency and cost-effectiveness.
- However, the industrial isolation of citric acid was initially carried out in Italy in 1890. The citrus fruit company employed hydrated lime, also known as calcium hydroxide, as a means to induce the precipitation of calcium citrate within the juice.
- Calcium citrate was isolated and subsequently converted back into its acidic form. This acidic form was then diluted using sulfuric acid.
Chemical Formula of Citric Acid
- Citric acid is typically categorized as a tribasic acid in scientific classification. To obtain acidic salts of Citric acid, it is imperative to carefully adjust the pH before commencing the crystallization process of the compound.
- One example of an acidic salt that can be derived from this particular acid is Sodium citrate.
- The chemical formula of citric acid is C6H8O7. Naturally occurring citric acid crystals are typically observed in a white color, a characteristic that is commonly observed on the surface of sour candies.
Synthesis of Citric Acid In Laboratory
- Citric acid exhibits remarkable versatility as a precursor to a plethora of diverse organic compounds.
- The process of dehydration yields itaconic acid and its corresponding anhydride.
- Itaconic acid anhydride can be used to make citraconic acid by going through a process called thermal isomerization. The itaconic acid anhydride that is necessary can be obtained through the process of dry distillation of citric acid.
- The process of synthesizing aconitic acid involves the dehydration of citric acid through the utilization of sulfuric acid as a catalyst.
(HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O- The decarbonylation of citric acid in fuming sulfuric acid is an alternative technique utilized for the synthesis of acetone-dicarboxylic acid.
How to Prepare Citric Acid at Home
- Step 1: Initially, it is imperative to procure a substantial quantity of lemons. To obtain a sufficient quantity of crystallized citric acid, it is recommended to use 450 ml of lemon juice. Please assess the acidity of the lemon juice by employing a pH strip. The desired outcome should ideally fall within the range of approximately 2 to 3 on the pH scale.
- Step 2: Proceed to the second step by introducing a small quantity of eyedrop solution, comprising 10% sodium hydroxide. Subsequently, reevaluate the solution’s properties. Please transfer the solution to another glass using the coffee filter. Additionally, it is advisable to conduct a visual inspection for the presence of any particulate matter within the solution that has been transferred into a fresh flask.
- Step 3: Proceed by adding 28 grams of Calcium Chloride to 70 ml of distilled water. Subsequently, merge both solutions into a single glass vessel and apply heat.
- Step 4: Once again, proceed to employ a coffee filter in order to separate the solution and extract the calcium citrate from it. Next, extract the Calcium citrate and combine it with a solution of heavily diluted sulfuric acid. Proceed by thoroughly stirring the mixture. Please separate the solution by using a filtration process and proceed to transfer the citric acid into a designated container, such as a beaker, for storage.
- Step 5: Apply moderate heat to the solution in order to facilitate the evaporation of water from the beaker. Afterward, proceed to separate the Citric acid by means of filtration and allow it to cool in a suitable container.
Properties of Citric Acid
- Citrate, colloquially known as citric acid, bears the distinguished systematic IUPAC nomenclature of 2-Hydroxypropane-1,2,3-tricarboxylic acid.
- The density of Citric acid is recorded to be 1.66 grams per cubic centimeter.
- The density of a chemical compound is a fundamental and pivotal property, as it elucidates the extent to which it can intermingle with a given substance.
- The molecular weight of Citric acid, existing in its monohydrate manifestation, is precisely 210.14 grams per mole.
| Citric Acid | C6H8O7 |
| Molecular Weight | 192.124 g/mol |
| Melting Point | 153 °C |
| Boiling Point | 310 °C |
| Density | 1.66 g/cm³ |
- Citric acid is classified as a weak organic acid.
- The molecular formula for citric acid is C₆H₈O₇.
- The compound is characterized by its lack of color, odor, and taste, presenting as a crystalline form.
- The crystalline structure of citric acid is classified as monoclinic.
- The molar mass of citric acid is 192.124 grams per mole.
- The density of the substance is 1.665 grams per cubic centimeter.
- The boiling point of citric acid is recorded to be 310°C, whereas its melting point is measured at 153°C.
- In addition to water, this substance exhibits solubility in ether, alcohol, and acetone.
- At ambient temperature, it is present in the form of a white crystalline powder.
- Upon reaching a temperature exceeding 175°C, the substance undergoes decomposition, resulting in the release of carbon dioxide and water.
- The chemical equation for the reaction is as follows:
C₆H₈O₇ + heat → CO₂ + H₂O.Advantages of Citric Acid
- Citric acid exhibits the remarkable ability to form a diverse array of metallic salts, as well as intricate complexes, with esteemed elements such as copper, iron, manganese, magnesium, and calcium. This characteristic of citric acid is widely acknowledged as a notable advantage, thereby conferring benefits upon its utilization.
- The utilization of Citric Acid as a sequestering agent for anticoagulant blood preservatives and industrial processes can be attributed to the presence of these salts. Furthermore, the concept of antioxidant properties in fats and oils is predicated upon their ability to mitigate metal-catalyzed oxidation through the process of chelation, specifically targeting trace amounts of metals, such as iron.
- The utilization of it as a seasoning can be divided into two segments. There are two primary reasons for the appeal of this substance. Firstly, its acidity is characterized by a minimal lingering sensation. Secondly, it possesses the ability to enhance various flavors.
- A novel methodology has been devised to eliminate sulfur dioxide from exhaust gases emitted through pipes. This innovative approach involves employing citric acid as a scrubbing agent, which forms a complex molecule that reacts with hydrogen sulfide, resulting in the production of a naturally occurring sulfur-recovering citrate compound. This phenomenon has the potential to assume a heightened level of significance in light of escalating environmental pressures.
- The employment of triethyl, butyl, and acetyl tributyl esters, which are derived from citric acid, as plasticizers in the realm of plastic films, is a commonly observed phenomenon. Instead of resorting to the use of citric acid, the application of mono stearyl citrate as an antioxidant in oils and fats is being observed.
Application of Citric Acid Powder
Citric Acid is obtained in the form of a finely powdered substance, exhibiting a pristine white hue. It facilitates the enhancement of renal well-being, addresses any afflictions pertaining to the pharynx, and effectively mitigates the presence of cutaneous blemishes.
Several notable applications of citric acid include:
- The utilization of citric acid as both a flavor enhancer and a preservative in various food products warrants contemplation.
- Citric Acid, a ubiquitous component in various consumables such as beverages and soft drinks, is commonly employed in the realm of food production.
- Citric acid, a substance commonly employed in the realm of culinary applications, finds itself not only utilized as a food additive but also plays a pivotal role in the confectionery industry, owing to its inherent tartness.
- Sour confections often incorporate the utilization of powdered substances of a pale hue.
- Certain ice cream manufacturers employ it as an emulsifying agent to deter the aggregation of lipid globules.
Application of Citric Acid in Cosmetics
- The utilization of deceased epidermal cells as facial masks can be regarded as an advantageous consequence of citric acid application.
- When citric acid is applied in cosmetic formulations, it demonstrates a notable capacity to improve skin complexion and stimulate the maturation of skin cells. Consequently, this can lead to a reduction in the visibility of wrinkles and acne scars.
- The citrus extract is commonly used in cosmetic formulations to adjust and regulate pH levels. This practice is known as rectification.
- It finds application in various cleansing agents, including cleansers, body washes, nail cleansers, facial chemicals, shampoos, and an assortment of beauty care formulations.
Application of Citric Acid in Cleaning
- Citric acid is commonly employed in soap production and as a water conditioner in cleansers due to its organic acid nature, as well as it’s chelating and buffering properties.
- The weak organic acid properties of citrus extract make it a dependable water conditioner.
- This method operates by effectively isolating various metal concentrations present in water, rendering it a highly suitable and environmentally-friendly alternative for treating hard water.
Application of Citric Acid in Cleaning Products
- Citric acid, when present in cleaning products, can be classified as a chelating agent.
- The process of eliminating citric acid The presence of limescale in evaporators and boilers can be considered one of the advantages of using citric acid.
- Citric acid is commonly utilized in soaps and laundry detergents due to its ability to soften water.
- Household cleaners commonly utilized in the kitchen and bathroom are formulated with a certain quantity of citric acid.
- One of the benefits of citric acid is its deodorizing properties.
Industrial Application of Citric Acid
- Acetic acid finds its application in a myriad of industrial sectors, including the esteemed realms of soap manufacturing, detergent production, electroplating processes, and the venerable art of leather tanning.
- Citric Acid serves as a preservative for the purpose of maintaining the integrity of stored blood, while also fulfilling the role of a buffer and antioxidant within the realms of the pharmaceutical and cosmetic sectors.
- The utilization of citric acid in various industries, such as food, feed, pharmaceutical, and polymer, has been discovered through extensive research on fungal-derived acids that possess significant commercial potential.
Health Effects of Citric Acid
- While citric acid can have health benefits, excessive exposure to it can lead to various negative effects such as increased stomach acidity, tooth decay, muscle twitching, numbness, or cramps. Additional potential side effects of citric acid include
- Prolonged contact with citric acid on the skin may result in irritation, swelling, the development of hives, and other related complications.
- It has the potential to impact both heart rate and mood fluctuations.
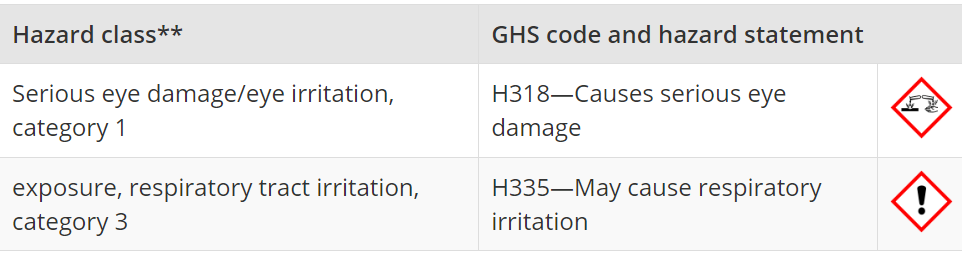
- Exposure to this substance may cause ocular irritation and potential damage to the eyes.
- Nausea or vomiting may be experienced as a potential side effect when orally consuming medications that contain citric acid.
- Certain adverse effects associated with citric acid may include diarrhea, convulsions, chest discomfort, dizziness, fatigue, cognitive impairment, and other related symptoms.
Citric acid is a naturally occurring compound found in various citrus fruits. Citric acid is not only present in citrus fruits but it can also be found in small amounts in all plants and animals. Numerous other products also contain citric acid. However, it should be noted that these items contain citric acid derivatives that are produced synthetically or through artificial means rather than being sourced naturally. Citric acid does not possess inherent antioxidant properties; however, it does augment the antioxidant capacity of numerous food products. It offers numerous advantages in a variety of ways. However, excessive utilization of it can also result in certain adverse effects. It is advisable to implement certain precautionary measures.
Video on Citric Acid
References
- https://pubchem.ncbi.nlm.nih.gov/compound/Citric-Acid
- https://www.webmd.com/diet/what-is-citric-acid
- https://www.acs.org/molecule-of-the-week/archive/c/citric-acid.html
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/ citric-acid
- https://byjus.com/chemistry/uses-of-citric-acid/
- https://www.chemicals.co.uk/blog/what-is-citric-acid
- https://www.britannica.com/science/citric-acid
- https://www.vedantu.com/chemistry/citric-acid
- https://www.toppr.com/guides/chemistry/aldehydes-ketones-and-carboxylic-acids/citric-acid/
- https://www.chemicalsafetyfacts.org/chemicals/citric-acid/

