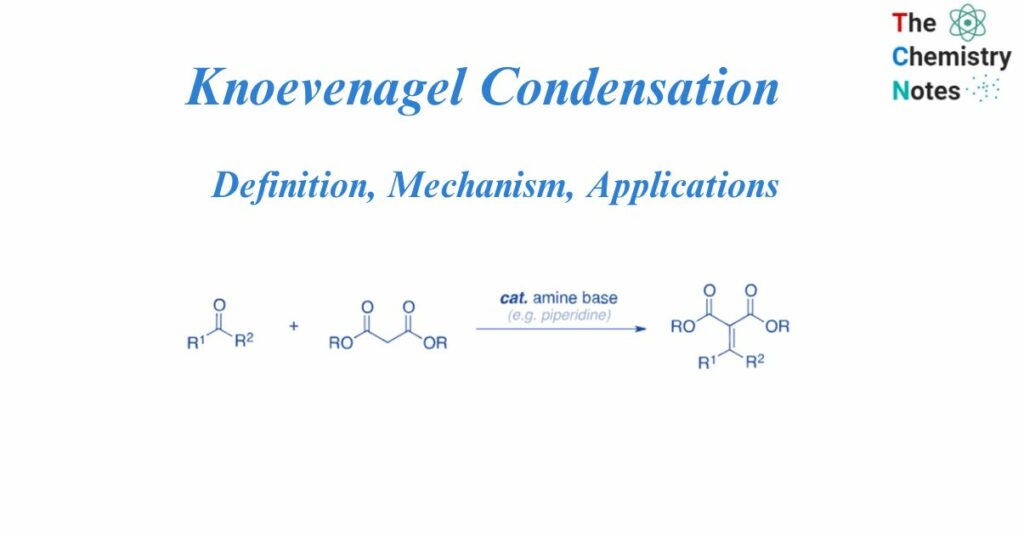
Knoevenagel condensation is the nucleophilic addition of an active hydrogen compound to a carbonyl group, followed by a dehydration process that eliminates a molecule of water. The result is frequently an alpha, beta conjugated compound.
The Knoevenagel process is a variant of the Aldol condensation reaction. For the synthesis of electrophilic olefins from active methylene and carbonyl molecules, the Knoevenagel reaction is widely recognized.
What is Knoevenagel condensation?
The Knoevenagel condensation is an organic reaction that uses an amine base as a catalyst to convert an aldehyde or ketone and activated methylene to a substituted olefin. The reaction begins with the base deprotonating activated methylene to produce a resonance-stabilized enolate. In addition, the amine catalyst combines with the aldehyde or ketone to generate an iminium ion intermediate, which is subsequently attacked by the enolate. The base deprotonates the intermediate compound to produce another enolate, while the intermediate’s amine is protonated. The amine base is later released, the catalyst is regenerated, and the final olefin product is produced.

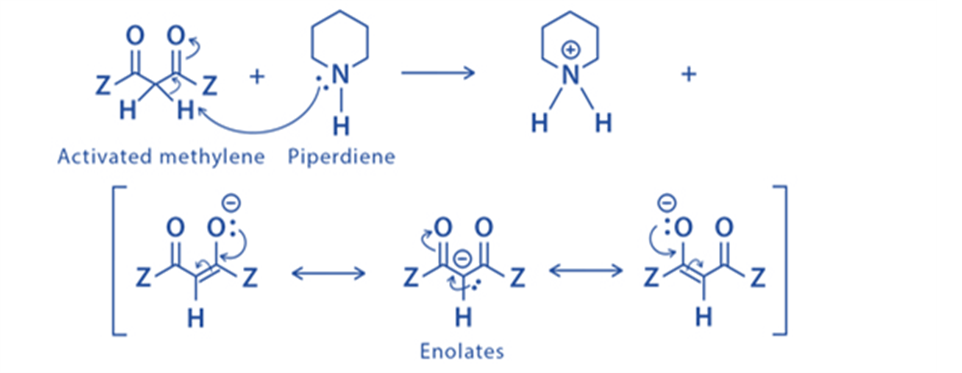
Mechanism of Knoevenagel condensation
Step 1: The activated methylene is deprotonated by the base (piperidine), yielding a carbanion that is resonance stabilized via the enolate ion.
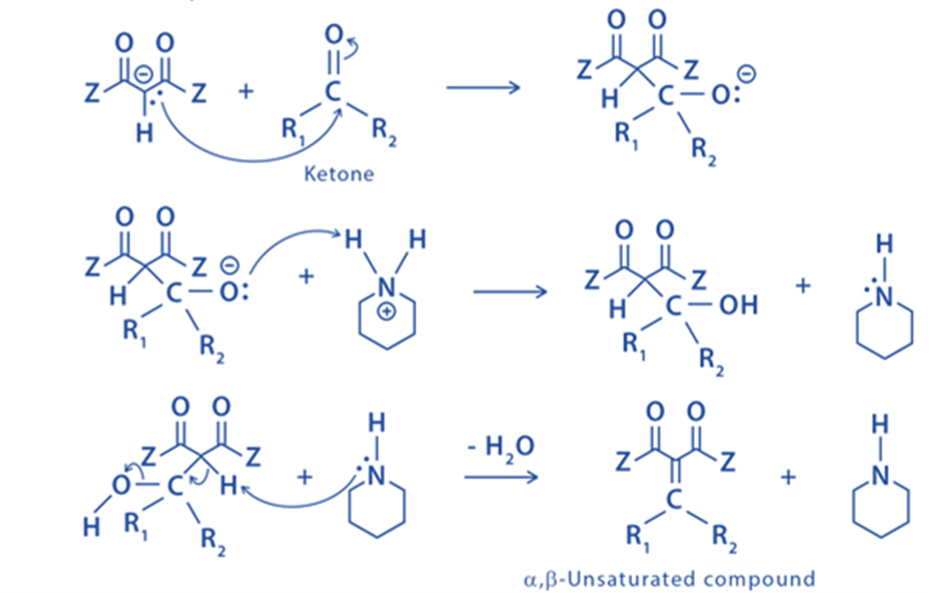
Step 2: The enolate ion undergoes a nucleophilic attack on the carbanion belonging to the carbonyl of the ketone.
Applications of Knoevenagel condensation
- The Knoevenagel condensation is an important step in the commercial manufacturing of lumefantrine, an antimalarial medication.
- The Knoevenagel reaction is utilized in the synthesis of conjugated enones, which are important intermediates in a variety of processes.
- A Knoevenagel reaction step is present in the Hantzsch pyridine synthesis, the Gewald reaction, and the Feist—Benaryfuran synthesis. The reaction was also responsible for the discovery of CS gas.
- Arylidene cyanoacetates and arylidene malononitriles were synthesized by the Knoevenagel condensation of various aldehydes with ethyl cyanoacetate and malononitrile, respectively, at room temperature or under pressure using imidazole as a catalyst.
- Coumarin, indole, quinoline, stilbenes, and other fascinating chemicals have been synthesized using this process.
- Knoevenagel’s utilization of primary and secondary amines and their salts as catalysts established a new platform for amine catalyst research.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
