Aldol condensations are significant in the synthesis of organic compounds because they offer a reliable method for forming carbon-carbon bonds. One of the crucial reactions involving carbonyl compounds (i.e. aldehydes and ketones) is the aldol condensation reaction. The Robinson annulation reaction sequence, for example, includes an aldol condensation; the Wieland-Miescher ketone product is a key starting material in many organics synthesizes. In its most common form, it entails the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, also known as an “aldol” (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals.
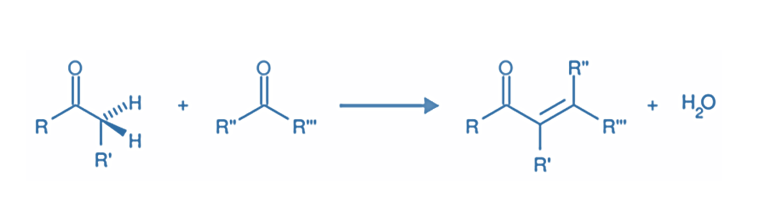
In organic chemistry, an aldol condensation is a condensation reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxy aldehyde or β-hydroxy ketone (an aldol reaction), which is then dehydrated to give a conjugated enone.
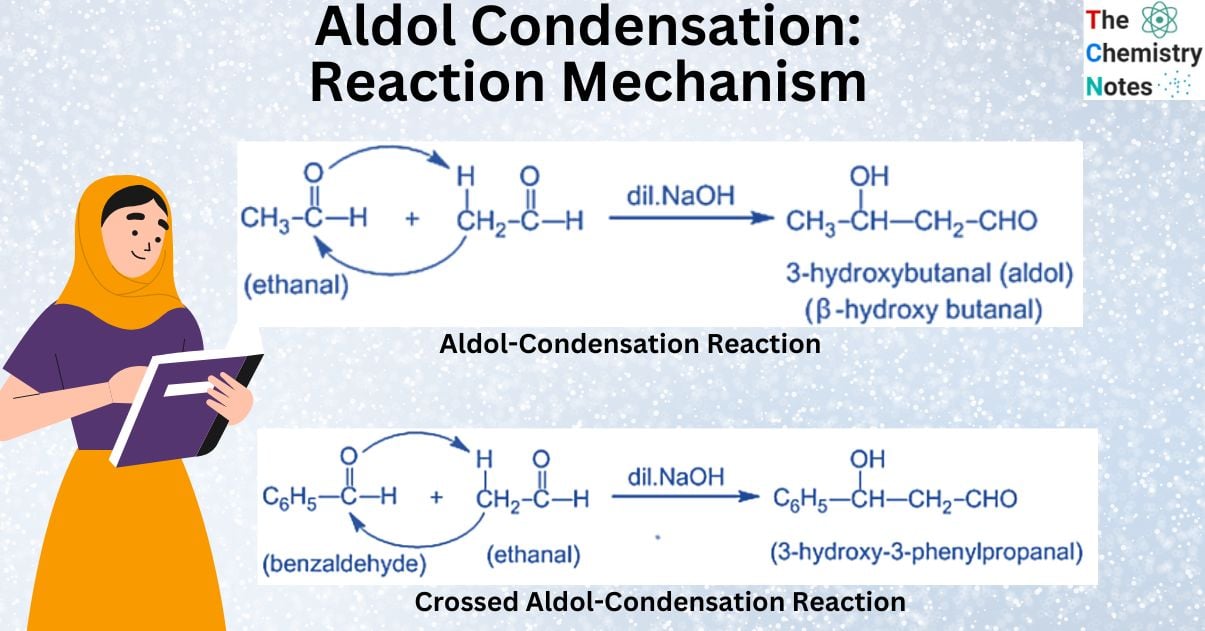
What is Aldol Condensation Reaction?
Aldol condensation reaction is the condensation of two aldehyde or ketone molecules with at least one hydrogen atom each in the presence of a diluted alkali to produce a hydroxylated form of the respective compound.
Aldehydes and ketones undergo an aldol reaction if they contain at least one α-hydrogen. As a result, the reaction takes place in the presence of an alkali (dilute). The dilute alkali acts as a catalyst, assisting in the formation of β-hydroxy aldehydes or aldol and β-hydroxy ketones or ketol. This reaction is referred to as aldol condensation.
Aldol condensation reaction does not take place with aldehydes and ketones that don’t contain any α – hydrogen atoms, such as HCHO, (CH3)3CCHO, C6H5CHO, etc.
The reaction gets its name from two different functional groups, aldehyde and alcohol, which are both present in the reaction. β-hydroxy aldehydes or aldol and β-hydroxy ketones or ketol easily lose water molecules to form, β-unsaturated carbonyl compounds. The products of this reaction caused by ketones are known as ketol. However, because their properties are similar to those of aldehydes, the reaction is known as aldol condensation.
Mechanism of Aldol Condensation Reaction
In the organic process known as aldol condensation, a carboxyl compound and an enolate ion combine to create either a hydroxy aldehyde or hydroxy ketone.
The water molecule is deprotonated by the alkoxide, resulting in hydroxide and -hydroxy aldehyde.
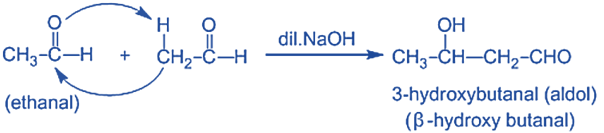
Step-I: In this step, an alkali hydroxide ion gives a carbanion (i.e. enolate ion) by removing a proton from the α – carbon of one molecule of ethanal. Since hydrogen acts as a base, it moves the acidic a-hydrogen and creates the reactive enolate ion. You can think of this reaction as an acid-base reaction.
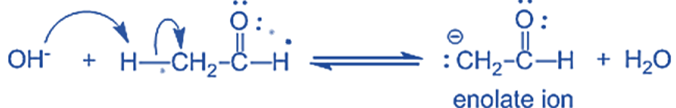
Step II: In this step, an alkoxide ion is created by the nucleophilic addition of an enolate ion to the carbonyl carbon of the second molecule of ethanal. This attack produces an alkoxide intermediate and is a nucleophilic addition reaction.
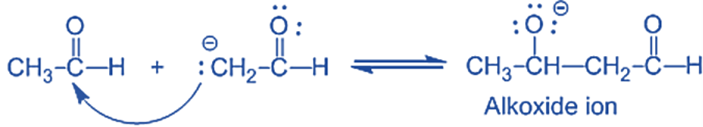
Step III: The alkoxide ion accepts a proton from water in this step to form β – hydroxy aldehyde (aldol).
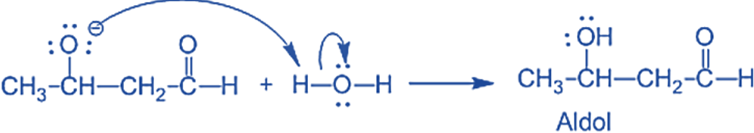
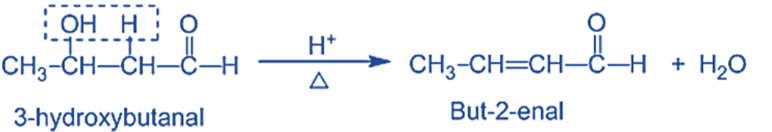
Step IV: Dehydration of aldol products- When heated with diluted acids, the aldol condensation product undergoes dehydration to produce α, β- unsaturated aldehydes or ketones.
Crossed Aldol Condensation Reaction
A crossed aldol condensation is an aldol condensation between two distinct carbonyl compounds. If both of the carbonyl compounds contain α-hydrogen, crossed aldol condensation cannot be used in a laboratory because a mixture of products is produced. It is helpful, though, if one of the carbonyl compounds lacks α -hydrogen and cannot undergo self-condensation. Benzaldehyde, for instance, can be used with other aldehydes and ketones that contain α-hydrogen.
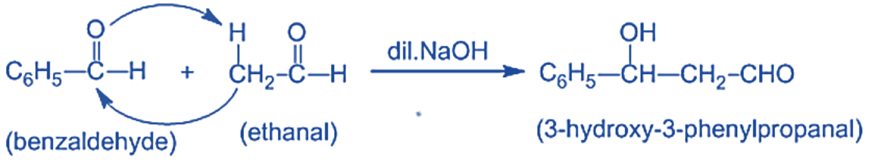
Mechanism of Crossed Aldol Condensation Reaction
Step I: In this step, an alkali hydroxide ion removes a proton from the α- carbon of ethanal to form a carbanion (i.e. enolate ion).
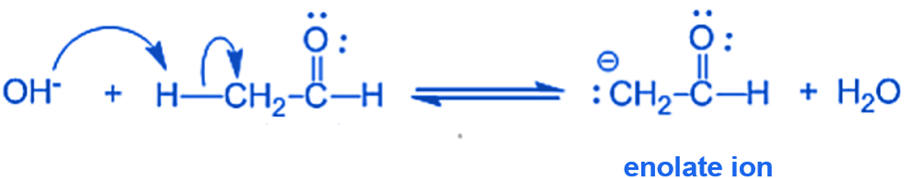
Step II: An alkoxide ion is formed by the nucleophilic addition of enolate ion to the carbonyl carbon of benzaldehyde.
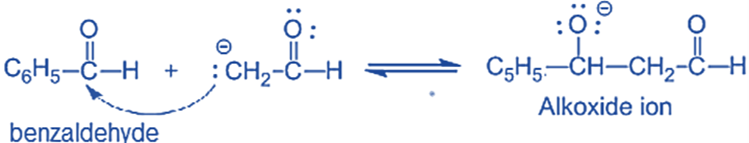
and Step III: The alkoxide ion accepts a proton from water in this step to form β-hydroxy aldehyde (aldol).
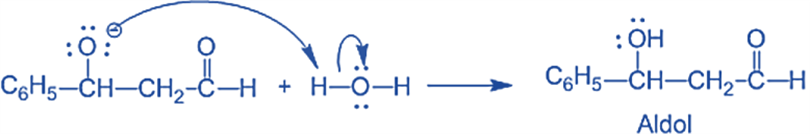
Applications of Aldol Condensation Reaction
Due to the fact that aldol products have two functional groups (-OH and -CHO), they can be used in a variety of reactions to produce a wide range of products. As an example:
- Catalytic hydrogenation of, α, β-unsaturated aldehydes or ketones derived from aldol dehydration yields saturated alcohols.
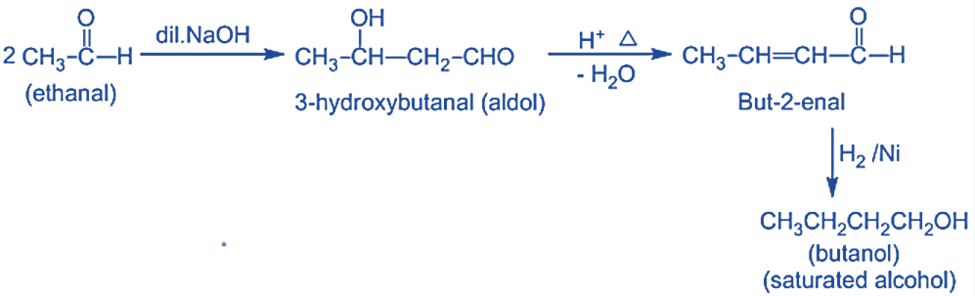
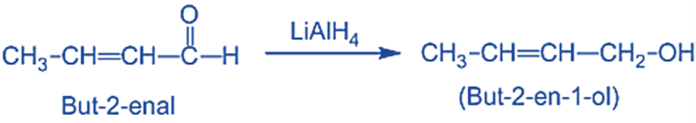
- Unsaturated alcohol is produced by reducing, α, β-unsaturated aldehydes or ketones with LiAlH4.
- The synthesis of sorbic acid, which serves as a food preservative.
- This reaction is used in the gluconeogenesis and photosynthesis processes to form a carbon-carbon bond.
- It has numerous applications and significance in the discipline of metabolism biochemistry, and thus has direct applications in the method of glycolysis, where it works to end the carbon-carbon end, which contradicts its role in the process of gluconeogenesis.
- It is used to make alcohol, isophorone, and diacetone.
- Also used as an intermediate in the perfume manufacturing process.
- Used in the production of pharmaceuticals.
- It is also used in the synthesis of pesticides.
- To make unsaturated ketones and chalcones (also known as aromatic ketones).
What is Alpha-Hydrogen?
To comprehend alpha hydrogen, we must first comprehend alpha carbon. The first carbon joined to the functional group is an alpha carbon. A carbonyl group is a functional group in the case of aldehydes and ketones. Alpha hydrogen is formed as a result of the functional group.
Because of the carbonyl group and its resonance stabilization mechanism, the hydrogen present on the alpha-carbon is slightly acidic in nature. Thus, alpha-hydrogen and its acidic nature are responsible for a variety of reactions.
References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://www.toppr.com/guides/chemistry/aldehydes-ketones-and-carboxylic-acids/reactions-due-to-alpha-hydrogen/#What_is_Alpha-Hydrogen
