Inductively coupled plasma atomic emission spectroscopy (ICP-AES) is a type of emission spectroscopy that uses Inductively coupled plasma to excite atoms or ions, resulting in electromagnetic radiation with a wavelength specific to a particular element. The intensity of this emission can be used to calculate the element content in the sample.
What is Inductively coupled plasma atomic emission spectroscopy?
Inductively coupled plasma atomic emission spectroscopy is an emission spectrophotometric technique that takes advantage of the fact that excited electrons emit energy at a specific wavelength when they return to their ground state after being excited by high-temperature Argon Plasma.
The temperature in plasma-based systems is significantly higher (10,000 K for Ar ICP), resulting in more effective excitation of atoms (usually greater than 90%) of around 60 elements, including several nonmetals. Because the extreme heat prevents polyatomic species from forming, the detection limits for several elements are increased.
The extreme heat of the plasma excites and, in many circumstances, ionizes atoms, and the emission of a photon occurs via resonance fluorescence (normal valance electron relaxation by photon emission). While plasma-based systems reduce many difficulties, they do not eliminate all interferences caused by the excitation and subsequent emission of spectral lines for each element in the sample, as well as the Ar injected to assist plasma production. The spectral overlap caused by these potential emissions is eliminated in current instruments using specialized sequential monochromators When compared to FAAS/FAES, ICP-AES has higher element selectivity, higher sensitivity, a larger dynamic range (particularly when compared to FAAS, which is limited by Beer’s law), lower detection limits, multi-element detection, and less matrix interferences.
Inductively coupled plasma atomic emission spectroscopy uses spectrometers that have a high throughput and can produce several reportable results in a single run. ICP-AES applies to practically every element, except for halogens and inert gases, and is particularly effective for refractory elements such as silicon, aluminum, barium, and others that perform poorly in flame AA.
Principle of Inductively coupled plasma atomic emission spectroscopy
An ICP source is made up of a flowing stream of argon gas that has been ionized by a radio frequency field. A water-cooled coil enclosing a quartz torch that maintains and restricts the plasma inductively couples this field to the ionized gas. In an appropriate nebulizer and spray chamber, a sample aerosol is created and transported into the plasma via a tube situated within the torch. The sample aerosol is directed into the ICP, where the constituent atoms are heated to temperatures ranging from 5000 to 10000oK. Because of the high temperature, molecules almost completely dissociate, resulting in a significant reduction in chemical interferences. The high temperature of the plasma efficiently stimulates atomic emission.
One or more optical spectrometers record the radiation released by the excited atoms, and when calibrated against standards, the approach offers a quantitative study of the original sample.
The primary feature of this process is that each element emits energy at certain wavelengths unique to its atomic nature. Each element has a different energy transfer for electrons when they return to their ground state because it depends on the electrical configuration of the orbital. The energy transfer is inversely proportional to the wavelength of electromagnetic radiation. Although each element emits energy at several wavelengths, the inductively coupled plasma atomic emission spectroscopy technique typically selects a single wavelength for a given element.
The amount (concentration) of that element in the sample being analyzed determines the intensity of the radiation emitted at the specified wavelength. Thus, by knowing which wavelengths are emitted by a sample and the intensities of those wavelengths, the analyst can qualitatively and quantitatively identify the elements in the provided sample in comparison to a reference standard.
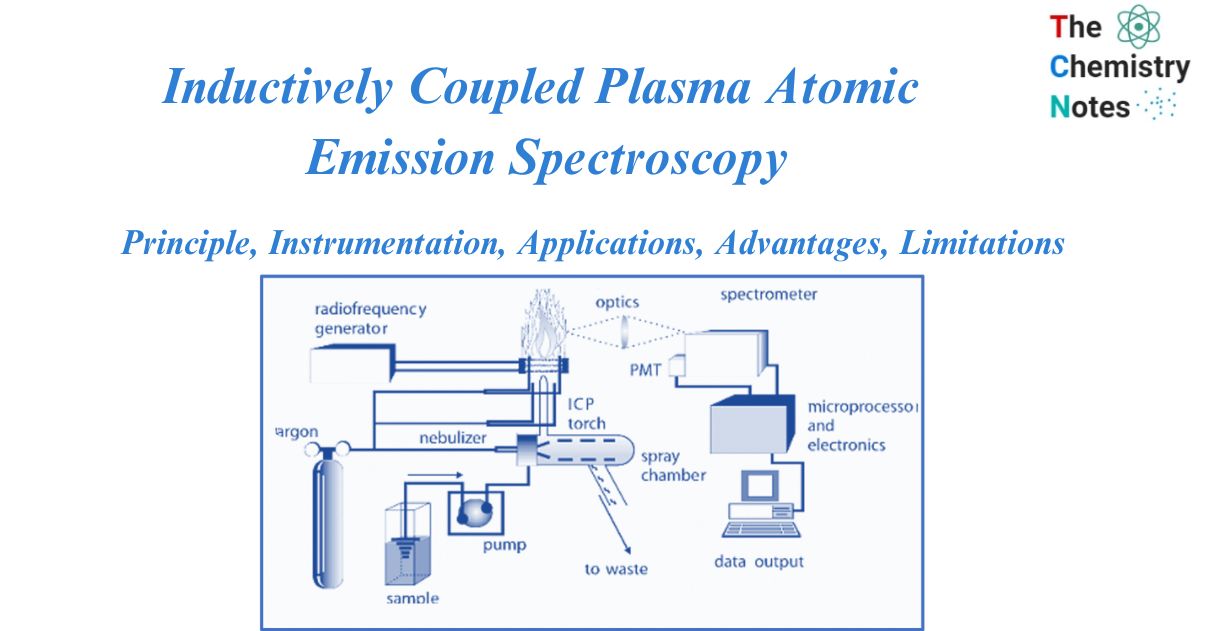
Instrumentation of Inductively coupled plasma atomic emission spectroscopy
Sample introduction
An Inductively coupled plasma atomic emission spectroscopy has successfully introduced all three states (solid, liquid, and gas).
Although both aqueous and nonaqueous solvents have been used, cations in solution are the most typically studied sample. A nebulizer is used to transform a liquid stream into an aerosol with particles 1-10 m in diameter for solutions. Direct liquid injection into the plasma would either extinguish the plasma or result in inappropriate atom desolvation, which would reduce the effectiveness of excitation and emission. In ICP, nebulizers are the most often utilized devices for introducing solution samples. The liquid sample is turned into an aerosol and transferred to the plasma using a nebulizer. Different nebulizers used for the sample introduction are:
Pneumatic nebulizers
Pneumatic nebulizers are the most often used form of nebulizer today. The flow of a nebulizing gas draws the sample solution via a capillary (the so-called Bernoulli effect). The spray chamber produces an aerosol that is divided by size, with smaller drops being delivered to the plasma.
Frit nebulizer
The sample solution is pushed to the frit membrane, which is constructed of a porous man-made substance that resembles coral in texture. Argon permeates the membrane, causing the sample to form an aerosol spray. Frit nebulizers can achieve efficiencies of up to 90% when the excess sample is drained. A wash solution input is included in the system to clean the frit membrane and prevent memory effects.
Ultrasonic nebulizer
A piezoelectric crystal vibrates at ultrasonic frequencies (50 kHz to 4 MHZ) in this device, and the sample is pushed to the crystal via a tiny plastic tube. The crystal vibrations force the droplets to split up into smaller particles, which are then transferred to the plasma. Larger drops of aerosol are drained.
Hydride generation
For some elements, hydride generation is a particularly successful sample introduction approach such as arsenic, bismuth, germanium, lead, antimony, selenium, tin, and tellurium
In this procedure, the sample is dissolved in a dilute acid solution and combined with a reducing agent, often a sodium borohydride solution in a dilute sodium hydroxide solution. The reaction of sodium borohydride with acid results in the production of hydrogen. The hydrogen subsequently reduces the metal ions in the analyte to hydrides. The following are some of the benefits of the Hydride generation technique:
- physical separation of the analyte from potential matrix interfering concomitants,
- higher efficiency than traditional pneumatic nebulization,
- ease of automation when used with flow injection techniques.
Plasma
The majority of Inductively coupled plasma atomic emission spectroscopy uses argon as the plasma gas. The following are some of the most advantageous features of the argon ICP source:
• high temperature (6000-8000 K),
• high electron density
appreciable degree of ionization for many elements,
• simultaneous multielement capability (over 70 elements including P and S),
• low background emission and relatively low chemical interference,
• high stability leading to excellent accuracy and precision,
• application to refractory elements, and
• cost-effective analysis.
However, there are other interesting applications of mixed-gas plasmas, including the entire replacement of argon to increase analytical figures of merit. The addition of other gases (e.g., H2, N2, O2, halocarbon gases, He, and other noble gases) to the composition of the auxiliary gas or nebulization gas might increase plasma characteristics such as thermal conductivity, electron number density, and plasma temperature.
Spray Chamber
A spray chamber is located between the nebulizer and the flame. The spray chamber’s principal function is to eliminate big droplets from the aerosol. The additional objective is to smooth out pulses caused by solution pumping during nebulization. It is intended to allow droplets with a diameter of 10 micrometers or less to pass through to the plasma.
Touch unit
An ICP’s torch unit is used to generate and sustain the plasma. A plasma is an electrically conducting gaseous mixture that contains enough cations and electrons to sustain conductance. The torch is encircled by a high-energy induction coil that is linked to a radio frequency generator, resulting in a massive electrical discharge in the torch.
The creation of heat is caused by resistance in the transport of electrons and argon gas through quartz tubes. The sample is delivered into the argon stream as a liquid aerosol or vapor.
This step allows the solvent to evaporate. Atomization and analyte excitation are the next steps.
Spectrometer
When the atoms in a sample are stimulated by plasma, they emit radiation at specified wavelengths. There are no two elements that emit radiation at the same wavelengths. The spectrometer’s role is to convert the emitted radiation from the plasma into wavelengths. Each wavelength will then be sent to a detector. These detectors are linked to a computer for data processing.
Applications of Inductively coupled plasma atomic emission spectroscopy
- Plasma sources have a high concentration of characteristic emission lines, making them helpful for both qualitative and quantitative elemental investigation.
- Inductively coupled plasma atomic emission spectroscopy is a very useful method for analyzing many heavy metals, metalloids, and even nonmetals at ppm to ppb levels.
- Inductively coupled plasma atomic emission spectroscopy is used to test for arsenic in food and trace elements bound to proteins.
- Inductively coupled plasma atomic emission spectroscopy techniques can be used to analyze agricultural products and foods.
- It is used in clinical analysis to determine metals in biological fluids (blood, urine).
- The determination of trace metals and other elements in waterways, soils, plants, composts, and sludges is part of the environmental analysis by using Inductively coupled plasma atomic emission spectroscopy.
- Inductively coupled plasma atomic emission spectroscopy is employed in medicines to analyze residues of catalysts and toxic metals (Cd, Pb, etc.).
- It is employed in forensic science to examine gunshot powder residue and toxicological examinations (e.g., thallium (Tl) determination).
Advantages of Inductively coupled plasma atomic emission spectroscopy
- Many elements are excited at the same time by the plasma.
- The analyst is not limited to analytical lines involving ground state transitions, but can also choose lines involving first or second ionisation states. The ion lines provide the best detection limits for the elements Ba, Be, Mg, Sr, Ti, and V.
- The plasma’s high temperature ensures the complete breakdown of chemical compounds (including refractory compounds) and inhibits the production of other interfering compounds, such as oxides, thus removing matrix effects.
- Samples in either liquid or solid state can be analyzed by Inductively coupled plasma atomic emission spectroscopy.
- It is highly specific.
- The ICP torch provides a chemically inert environment and an optically thin emission source that unless at very high concentrations, is not subject to self-absorption.
- The excitation and emission zones are separated spatially, resulting in a reduced background.
- Argon is inert, meaning it does not react with the sample.
- Low background leads to low detection limits, which are often in the ppb range.
- A large number of elements can be measured by using Inductively coupled plasma atomic emission spectroscopy.
Limitation of Inductively coupled plasma atomic emission spectroscopy
- Different viscosities can influence sample uptake of Inductively coupled plasma atomic emission spectroscopy.
- Matrices can alter the character of plasma. Torch can be attacked by certain matrices.
- Matrices can have overlapping spectral components.
- Physical changes in the optical system or the arrangement of the plasma might cause instrument readings to drift over time. To estimate and adjust for this drift, standards must be run at the start and end of each run.
References
- https://www.whitman.edu/chemistry/edusolns_software/FAAS_ICP_2017/CH4_ICP-AES_2017.pdf.
- http://chemistry.univer.kharkov.ua/files/icp.pdf.
- https://www.slideshare.net/ParimiAnuradha/inductively-coupled-plasma-atomic-emission-spectroscopy-65578961.
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
