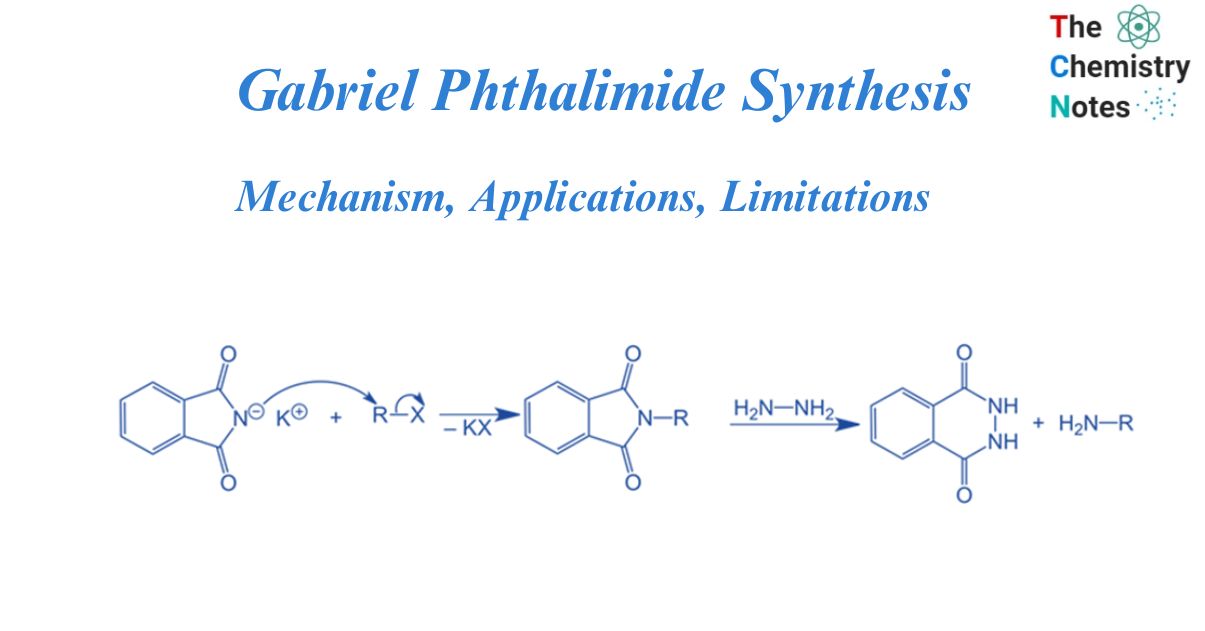
The Gabriel phthalimide synthesis is a chemical reaction that converts primary alkyl halides to primary amines. It is a nucleophilic substitution process that is mostly utilized to produce primary amines. Siegmund Gabriel, a German scientist, and his friend James Dornbush discovered the Gabriel Synthesis in 1887. It is a straightforward and effective process for producing a wide range of primary amines from easily available starting materials. Additionally, the process of the reaction takes place at moderate conditions, so, no abrasive chemicals or high temperatures are needed. As phthalimide is employed in this reaction along with a base, is known as Gabriel phthalimide synthesis. It is also known as Gabriel synthesis.
What is Gabriel’s phthalimide synthesis?
In the presence of strong bases like KOH, and NaOH, alkyl halide reacts with pthalamide to give N-alkyl pthalimide. N-alkyl phthalimide on hydrolysis produces primary amine. This is known as Gabriel phthalimide synthesis.
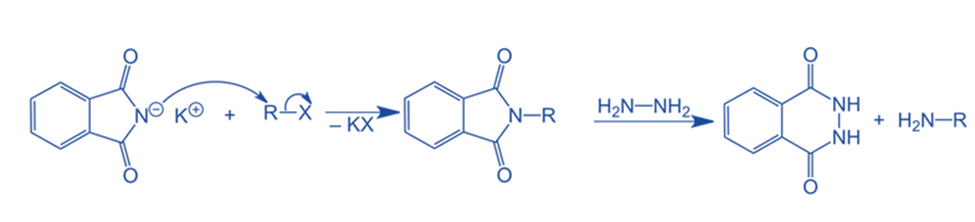
Mechanism of Gabriel phthalimide synthesis
Step I: The reaction begins with the conversion of phthalimide to potassium phthalimide via the addition of an alkali base such as KOH or NaOH. Deprotonation of nitrogen occurs when protons are removed from the nitrogen of phthalimide.
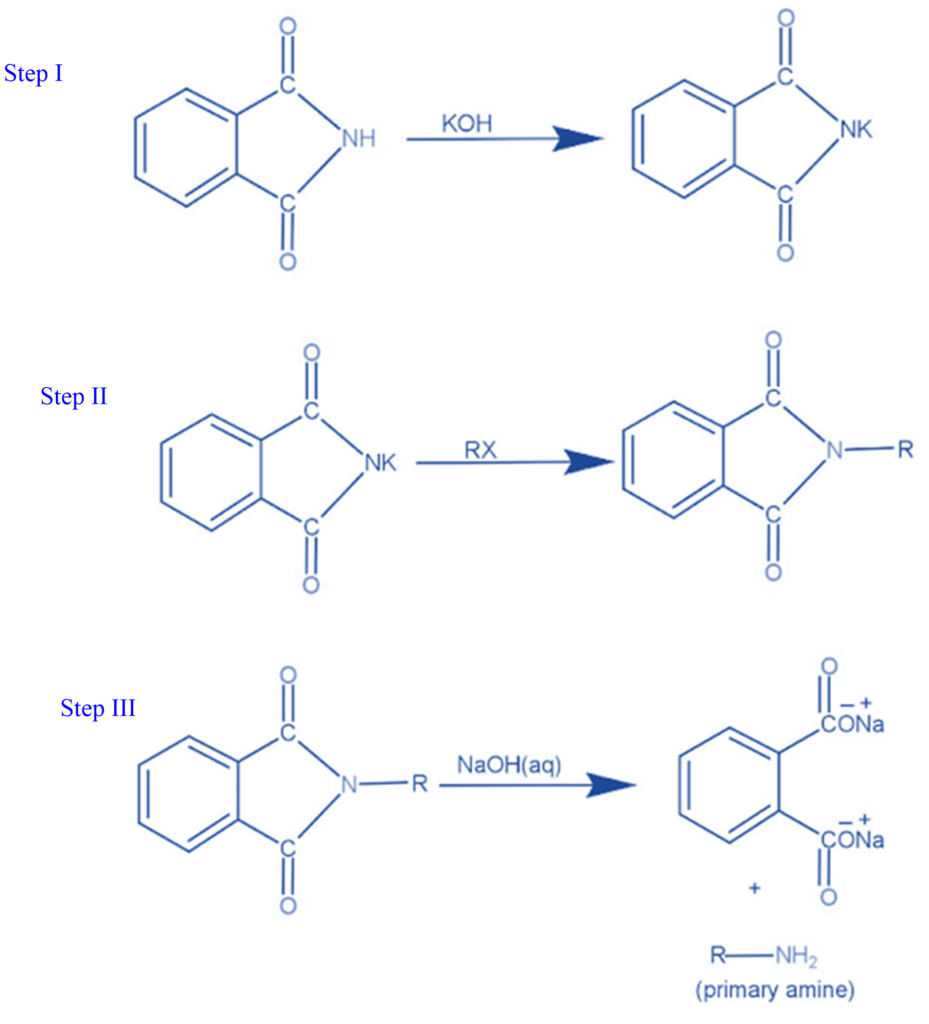
Step II: With potassium phthalimide, the Gabriel phthalimide synthesis continues. Deprotonation causes the nitrogen in potassium phthalimide to acquire a negative charge. This negatively charged nitrogen can now operate as a nucleophile, reacting with alkyl halide via the bimolecular nucleophilic substitution process, also known as the SN2 reaction. Gabriel phthalimide’s nucleophilic nitrogen attacks the electrophilic carbon of alkyl halide. The potassium ion from KOH reacts with the halogen of an alkyl halide to create KX. N-alkyl phthalimide is formed when the alkyl group of alkyl halide attaches to nucleophilic nitrogen.
Step III: In the third stage, which is comparable to the base-catalyzed hydrolysis of esters except that the resulting bond is between nitrogen and the R group rather than oxygen and nitrogen, the R group joins with the nitrogen atom. The hydroxide ion in potassium hydroxide subsequently binds to the carbon atom, causing the N-alkyl phthalimide to dissociate and produce a primary amine.
Applications of Gabriel phthalimide synthesis
- The Gabriel synthesis has the fundamental advantage of preventing overalkylation.
- The Gabriel reaction has been expanded to include sulfonamide and imide alkylation followed by deprotection to yield amines.
Limitations of Gabriel phthalimide synthesis
- The Gabriel phthalimide synthesis technique can only produce primary alkyl amines.
- A secondary or tertiary amine cannot be produced from it.
- As aryl halides do not undergo simple nucleophilic substitution, aryl amines cannot be synthesized using the Gabriel technique.
- The use of acidic/basic hydrolysis leads to a low yield, and the use of hydrazine can make the synthesis conditions relatively severe.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
